5551
A methodology to study brain specialization and integration during the information processing1Department of Physics, FFCLRP, University of São Paulo, Ribeirão Preto, Brazil
Synopsis
There is interest in understanding the functional integration of cognitive functions and effective therapeutic interventions as a strategy to improve cognitive deficits. However, uncertainty about how to proceed to address those issues remains. Therefore, this study aims to propose a methodology to assess brain specialization and integration during a cognitive task for future studies on the evaluation of therapeutic strategies. Our methodology provided a network model related to the performed task, that may serve as a reference for future investigations in clinical groups; and coupling parameters that may be used to evaluate the presence of adaptative neuroplasticity after cognitive training/rehabilitation.
Introduction and Purpose
Recently, there has been an upsurge of interest in understanding the functional integration of cognitive functions and effective therapeutic interventions as a strategy to improve cognitive deficits1. However, considerable uncertainty about how to proceed to address those issues remains. Functional brain mapping should include two fundamental principles of functional organization: specialization and integration2. The integration within and between specialized areas is mediated by the effective connectivity, whose evaluation over time may indicate the presence of associative neuroplasticity. The information processing speed (IPS) calls attention for its relationship with attentional deficits and its impairment in patients with traumatic brain injury, depression, dementia and multiple sclerosis3. Therefore, this study aims to propose a methodology to assess brain specialization and integration during a cognitive task for future studies on the evaluation of therapeutic strategies.Materials and Methods
The proposed methodology included five steps:
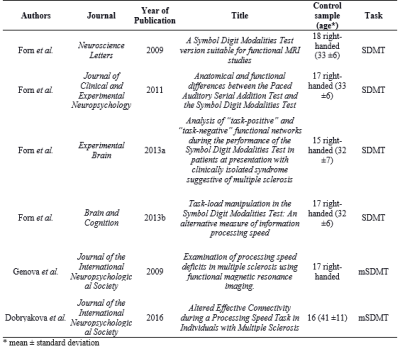
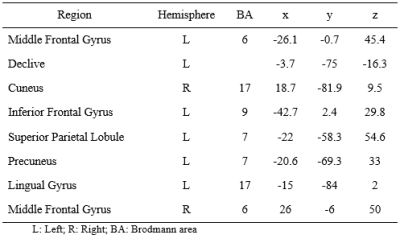
1. Meta-analysis to identify IPS-related brain regions. We performed a meta-analysis of six studies (Table 1) that assessed the IPS using functional MRI (BOLD-fMRI) and an adapted version of the Symbol Digit Modalities Test (SDMT). We used the ALE algorithm4, significance level for p-FWE<0.05, and created a 3D brain template for each region (Table 2).
2. fMRI experiment. 16 right-handed healthy subjects (7 women, 29.7±5.0 years) were recruited after the study approval by the Research Ethics Committee. MRI was acquired in a 3T system using a 32-channel head coil for signal reception. BOLD and anatomical images were acquired with usual sequences and parameters. The functional experiment consisted of six blocks of control (30 s each) intercalated by five blocks of SDMT task (30 s each). During the task blocks, a symbol was displayed (on a monitor) every 2 seconds and the participant was asked to associate the number corresponding to the displayed symbol based on a response key. During the control blocks, a number was displayed every 2 seconds and the participant was asked to quietly read the number.
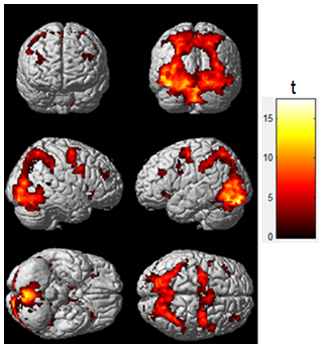
3. Mapping of IPS-related regions. Standard image preprocessing was performed using the SPM12 software5. The statistical parametric map was obtained using the General Linear Model (GLM) with a boxcar regressor convolved with a canonical hemodynamic response function (p-FDR<0.01). It was then superimposed to the templates of Figure 1 to obtain the mean time series of each region.
4. Functional connectivity (FC) analysis. We used the CONN toolbox6 to perform a bivariate correlation between the time series of each IPS-related region (p-FDR<0.0001). The information of functional location and integration was inserted into the effective connectivity analysis.
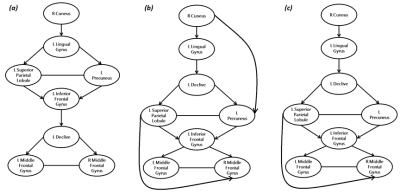
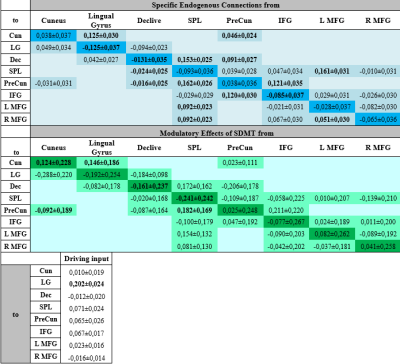
5. Effective Connectivity Analysis. To select the best network model among the hypothesized ones and provide the effective connectivity parameters, we created three models based on the meta-analysis results (Figure 2a), seed-to-voxel (Figure 2b) and ROI-to-ROI (Figure 2c) FC analysis. Intrinsic connections were considered within and between each region, and were considered to be modulated by the SDMT condition versus baseline. We used the Bayesian model selection (DCM-SPM12) to select the best network, and then the estimates of the parameters of this model (driving inputs, intrinsic connections and modulations) were submitted to group analysis with one-sample t-test (p<0.05).
Results
Functional maps showed activations in the frontoparietal network and the occipital cortex for individual and group analysis (Figure 1), which agree with the regions obtained with the meta-analysis (Table 2). Seed-to-voxel FC analysis showed the information propagating as in model 2 (Figure 2b). ROI-to-ROI analysis presented similar pattern, regarding the exclusion of the cuneus to precuneus connection (model 3 - Figure 2c). The most probable network architecture was represented by model 2, and its coupling parameters (in Hertz) are shown in Table 3.Discussions
The meta-analysis step provided important information about brain regions related to the task, helping the choice of regions to be inserted in the FC analysis the creation of a theoretical model. Obtaining the activation map allowed to verify the brain activated areas for the group and extract the regions to be inserted in FC analysis. FC patterns provided network structures involved in SDMT execution, solving the challenging question about hypothesizing network structures. Effective connectivity analysis provided a network structure model for IPS and parameters that may be used to analyze therapeutic strategies.Conclusions
Our proposed methodology provided a network model related to the performed task, may serve as a reference for future investigations of IPS in clinical groups; and coupling parameters that may be used to evaluate the presence of adaptive neuroplasticity after cognitive training/rehabilitation in longitudinal studies. Next steps include the better investigation of those parameters.Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES, Brasil.References
Figures