5530
Accounting for pCASL labelling efficiency variation in patients with low and high arterial blood flow velocities1Radiology & Nuclear Medicine, Academic Medical Center, Amsterdam, Netherlands, 2Institute of Neurology/Centre for Medical Image Computing, University College London, London, United Kingdom
Synopsis
Pseudo continuous arterial spin labelling (pCASL) suffers from reduced labelling efficiency in extreme flow conditions. In this work we investigated the sensitivity of labelling efficiency to velocity values measured in vivo to ascertain its variability over a clinically relevant range of velocities. We measured arterial blood velocity in the neck at the level of the labelling plane, and obtained simulated labelling efficiency values, which we found to differ significantly between high and low velocity populations. Changes in labelling efficiency induced by acetazolamide administration may have implications for future work using pCASL for cerebrovascular reserve assessments.
Introduction
While pCASL is the most widely used arterial spin labelling technique for non-invasive cerebral perfusion imaging, it generally suffers from lower labelling efficiency than the pulsed variant. This can be exacerbated by very low or very high velocity blood flow in the arteries where labeling occurs. Since perfusion imaging is often applied to elderly subjects with low arterial blood flow, or alternatively patient populations with high flow (e.g. sickle cell disease), there is a gap in our understanding of the extent to which the labelling efficiency varies in clinically relevant situations. In this work, we performed simulations of labelling efficiency of different pCASL implementations with respect to the velocity of the intraluminal spins being inverted during a pCASL experiment. We compared the velocities measured in the labelling plane using phase-contrast MRI (PC-MRI), and compared the corresponding labelling efficiencies between groups with varying flow conditions: elderly subjects, adults with sickle cell disease, and paediatric sickle cell disease patients, who are expected to have the highest velocities.Methods
Imaging: Thirty-two adults and thirty-five children with sickle cell disease (SCD), eleven healthy controls, sixty-eight elderly subjects undergoing pCASL studies for research at our lab were included. Studies were conducted under IRB-approval and images were acquired on 3.0 Tesla clinical MR systems (Intera/Ingenia, Philips Healthcare, Best, The Netherlands) with a 15/32-channel head coil and body coil transmission. Phase contrast (PC-)MRI images were acquired in a single slice positioned 90 mm below the centre of the pCASL imaging volume, with the following parameters: TR/TE=15/5 ms, FOV 230 x 230 mm, voxel size 0.45 x 0.45 mm, slice thickness 4 mm, flip angle 15°, VENC 80/140 cm/s adjusted for elderly subjects and SCD respectively, and a total scan duration of 60 seconds.
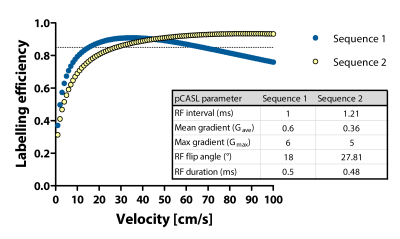
Simulations: Bloch equations were solved to test the effect of pCASL labelling parameters used in the various cohorts that were included, i.e Sequence 1 and 2, for a range of velocities (curves are provided in Figure 1).
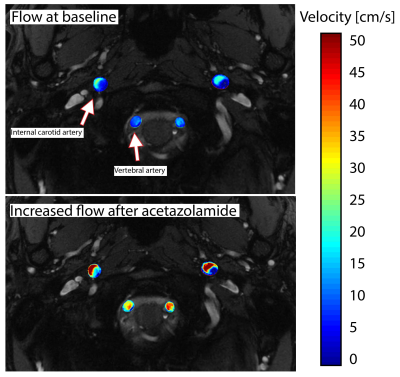
Post-processing: Phase offset correction and phase-unwrapping was performed in cases of velocity aliasing in the PC-MRI data. The internal carotid arteries (ICA) and vertebral arteries (VA) were segmented on the magnitude images using commercial software Mimics (Materialise, Leuven, Belgium). Data were masked in MATLAB (MathWorks, Natick, MA, US) using customised scripts. Mean and maximum velocity, flow, and lumen cross-sectional area were calculated for each vessel indicated by arrows in Figure 2. Labelling efficiency values for each vessel were chosen based on the maximum measured velocity (Vmax), assuming laminar flow. A weighted labelling efficiency was based on the percentage of volumetric flow contributed by each vessel.
Analyses: (Non)-parametric and paired tests were performed to test group-, region-, and ACZ-induced differences. P<0.05 was considered statistically significant.
Results
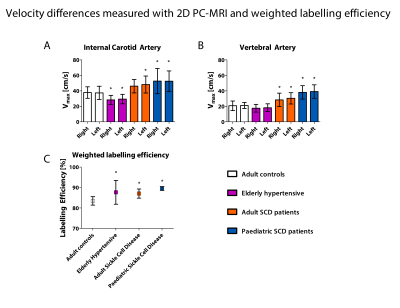
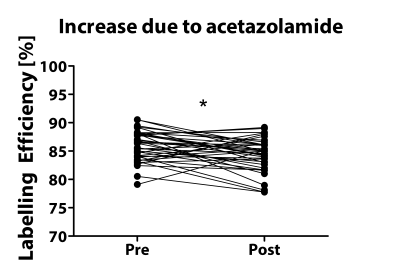
Phase contrast MRI results are shown in Figure 3 which shows significant differences in maximum velocity in the internal carotid and vertebral arteries between healthy adult controls, hypertensive elderly, and sickle cell disease groups. Volumetric flow rate was ~70% of the total flow for the ICA vessels and ~30% of the total flow in the VA vessels in all cohorts. Weighted labelling efficiency was significantly higher in all cohorts compared to adult healthy controls, as shown in 3c. Surprisingly, the elderly hypertensive cohort with the lowest velocity had higher mean labelling efficiency than the adult healthy controls, because they had been scanned with Sequence 1, which had high efficiency at low velocities, compared to Sequence 2 which had low efficiency at low velocities. Labelling efficiency increased significantly after acetazolamide administration due to increased velocity (Figure 4, p=0.002).Discussion
We found varying labelling efficiency sensitivity to velocity in four groups, where sickle cell disease and elderly had higher efficiency than the adult healthy controls. This was because the relationship between labelling efficiency and velocity was also sequence dependent, meaning labelling characteristics also played a role in determining if a certain velocity would result in high or low efficiency. Therefore, estimating and correcting for the efficiency may improve quantification of CBF from pCASL, because we have shown that there can be considerable changes in efficiency due to velocity. These findings have implications for cerebrovascular reserve experiments because velocity increases and, depending on the sequence used, may either increase, or decrease labelling efficiency.Conclusion
In conclusion, pCASL labelling efficiency can be estimated and accounted for in cases where velocity in the labelling region is expected to be much lower or higher than the optimal range.Future work is directed towards investigation of the effect of a correction for labelling efficiency in CBF quantification for cerebrovascular reserve studies.Acknowledgements
This work was supported by Dutch foundation “Fonds Nuts Ohra” grant no. 1303-055. Clinical trials.gov identifier: NCT02824406. DLT is supported by the UCL Leonard Wolfson Experimental Neurology Centre (PR/ylr/18575).References
S Aslan, F Xu, PL Wang, J Uh, U Yezhuvath, M van Osch, H Lu. ESTIMATION OF LABELING EFFICIENCY IN PSEUDO-CONTINUOUS ARTERIAL SPIN LABELING. Magn Reson Med. 2010 Mar; 63(3): 765–771.Figures