5520
Whole Brain Absolute CBF Measurements Using Ultra-Low GBCA DCE-MRI and Microsphere Model1Division of Informatics, Imaging and Data Science, The University of Manchester, Manchester, United Kingdom, 2Department of Neurosurgery, Salford Royal NHS Foundation Trust, Manchester, United Kingdom
Synopsis
Previous authors have attempted to utilize the microsphere model and a maximum gradient (MG) based approach to estimate cerebral blood flow from DCE MRI data, which, however, was not widely accepted. This study developed a new methodology based on the microsphere theory and “early time points” method for mapping absolute CBF, using ultra-low contrast agent dose T1W-DCE-MRI. The new method was assessed using both computer simulation and in vivo data analysis in a patient with GBM, demonstrating that the new method performed better than previous approaches, e.g. MG-based methods, while using much lower dose than perfusion methods based on DSC-MRI.
Introduction
The aim of the study is to develop and assess a new methodology, which is based on the microsphere theory1 and the “early time points” method (ET)2, for absolute CBF estimation using ultra-low dose (1/5 of standard) T1W DCE MRI. Using both Monte Carlo simulation and in vivo study in a patient with glioblastoma multiforme (GBM), we aim to test two specific hypotheses. Firstly, with the low dose compact bolus, the whole upslope segment prior to the first pass peak of the tissue concentration-time curves can be taken as the ET window. Secondly, when the effects of noise are considerable, an averaged concentration (AC) based method, especially when applied on data points located in a segment with higher ENR, is superior to a maximum gradient (MG) based method.Methods
Patient: The patient with GBM was imaged on a 3.0T Philips scanner. The imaging protocol includes 1) A low dose high-temporal resolution (LDHT) DCE MRI: ∆t = 1.0 s; 3 ml (~0.02 mmol/kg) of contrast agent (CA) administered by power injector at a rate of 3 ml/s, followed by a chaser administered at the same rate. 2) A DSC series, using a 3D PRESTO3.
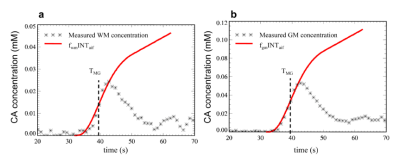
Microsphere model: In DCE MRI, the contrast agent can behave like a microsphere during ‘early time points window (ETW)’, in that no indicator that has arrived in an organ will have yet left it. The microsphere model can be therefore applied:
$$$C_{t}(t)=f\int_{0}^{t} C_{b}(t')dt'$$$ with t ϵ ETW, [1]
where the tissue CA concentration, Ct(t), is equal to the amount of CA delivered to 1 ml of tissue by time t, f is the perfusion term1 and Cb(t) is the arterial blood concentration. The MG method is then expressed as:
$$$ f=max(\frac{dC_t(t)}{dt})/max(C_{b}(t))$$$ [2]
Assessing the validity of the low dose T1W-DCE MRI data for using the microsphere model: Figures 1a and 1b demonstrate the assessment of whether Eq. 1 holds true for the DCE data from the patient with GBM.
CBF measurement: Five ways for absolute CBF evaluation were compared:
1) The classical MG (MGclassical) method by searching the greatest difference in tissue enhancement between two consecutive data points (i and i+1) on the rising bolus time course.
2) The slope method - The slope over the four points, [i-1, i, i+1, i+2] is used as the numerator in Eq. [2].
3) Averaged concentration referencing tMG (the ACrMG method), where tMG = (ti + ti+1)/2. The running average of three data points [i+1, i+2, i+3] was used as the value of Ct(t) in Eq. [1].
4) Averaged concentration referencing tpeak (the ACrPK method), where tpeak was the tissue peak time. The running average of three data points [ipeak-1, ipeak-2, ipeak-3] was used as the value of Ct(t) in Eq. [1]. For both ACrMG and ACrPK, t = tMG + 1.5(Δt) = tAIF_peak + 1.5(Δt) was used as the upper time limit in integration of Cb(t).
5) Based on the results from Monte Carlo error analysis of the above four methods (Fig. 3), we developed a new method, which takes the average of the CBF values obtained from the ACrMG and ACrPK methods, the ACcomb method.
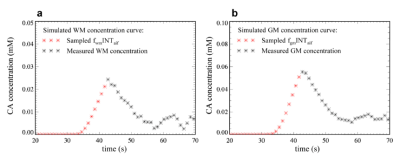
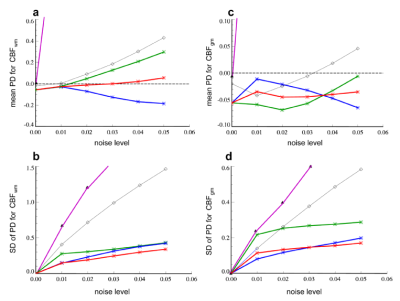
Monte Carlo simulation: Figures 2a and 2b show a simulation of the tissue concentration curves based on the microsphere model. Rician white noise with noise level (= standard deviation/ mean baseline signal) of 1%, 2%, 3%, 4% and 5% respectively was added to the simulated SI-time curves4. Absolute CBF values were calculated using the synthetic data sets to produce the so-called ‘measured’ values with the five estimation methods respectively. Percent deviations (PD) of the ‘measured’ values from the ‘true’ values were calculated as: PD = (measured – true)/true∙100%. 20000 repetitions were performed for each method to produce mean and standard deviation (SD) of PD for each CBF estimates.
Results
Figure 3 compares percent deviation in CBFWM and CBFGM derived from the five T1W-CBF methods, at various noise levels.
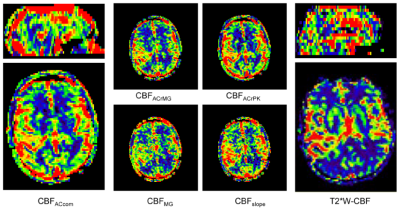
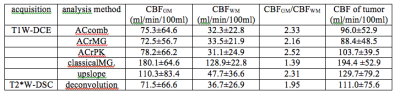
Figure 4 shows the CBF images derived using the T1W_ET methods and the DSC-deconvolution from the patient with GBM. The ROI mean CBF values of the GM, WM and tumor derived from various methods are listed in Table 1.
Both Monte Carlo simulation and in vivo study demonstrated that the AC methods performed better than upslope or MG methods.
Conclusion
The new method is able to provide reliable CBF values using a ultra-low dose DCE MRI acquisition. The ability to provide whole brain 3D coverage with a short acquisition time and low contrast agent dose makes this new method a promising technique for CBF measurement in clinical practice.Acknowledgements
This work was supported by CRUK [C8742/A18097]. This is a contribution from the Cancer Imaging Centre in Cambridge & Manchester, which is funded by the EPSRC and Cancer Research UK.References
1. Peters AM. Fundamentals of tracer kinetics for radiologists. Br J Radiol 1998;71(851):1116-1129.
2.. Kwong KK, Chesler DA. Early time points perfusion imaging: theoretical analysis of correction factors for relative cerebral blood flow estimation given local arterial input function. Neuroimage 2011;57(1):182-189.
3. Moonen C, Liu G, Van Gelderen P, Sobering G. A fast gradient-recalled MRI technique with increased sensitivity to dynamic susceptibility effects. Mag Res Med 1992;26:184-189.
4. Li KL, Zhu X, Zhao S, Jackson A. Blood-brain barrier permeability of normal-appearing white matter in patients with vestibular schwannoma: A new hybrid approach for analysis of T1 -W DCE-MRI. J Magn Reson Imaging 2017;46(1):79-93.
Figures