5504
The developmental dependence of the contrast between white and grey matter in spinal cord: the effect of non-aqueous species content1School of Chemistry, Tel Aviv University, Tel Aviv, Israel, 2SQITS/NICHD, NIH, Bethesda, MD, United States
Synopsis
Recently an imaging pulse sequence based on selective saturation of the water that drives magnetization transfer from the non-aqueous semi-solid part of the tissues was introduced. This technique allows the estimation of the fraction of the protons in the non-aqueous species. It separates the exchange processes from T1 in a simple way. In the current study, using this technique, we demonstrate changes with age of the non-aqueous species content in porcine spinal cord.
Introduction: Recently an imaging pulse sequence based on selective saturation of the water that drives magnetization transfer from the non-aqueous semi-solid part of the tissues was developed. This technique allows the estimation of the fraction of the protons in the non-aqueous species1. It separates the exchange processes from T1 in a simple way and does not require an elaborate computation like MTC2. Moreover, it is less sensitive to r.f. inhomogeneity occurring in selective inversion3. In previous studies it was demonstrated that the contrast between white (WM) and grey matter (GM) in spinal cord (SC) is affected by the non-aqueous species content1,2. Imaging, using ultra-short time-to-echo (UTE) of SC and of myelin extracts demonstrated that the contrast in SC is dominated by the non-aqueous species4, notably lipids and membrane proteins. Correlation of myelin extract concentration with the WM signal attributed to myelin was shown4. Previous studies, using non-MRI methods, have shown that myelin content is increasing with porcine age5. In the current study, using selective saturation of water followed by an imaging block and analyzing the results by suitable theoretical models, we demonstrate this age-dependence in porcine spinal cord.
Materials and Methods: Selective saturation (SelSat) was done by: 90°-crusher-90°-crusher-tLM-Imaging (90o -3ms, crushers were set to disable echo formation; MRI module was gradient echo (GE) with TE=2ms,TR=5s). The samples were spinal cords excised from 2 (weight 20kg) and 4 (weight 48 kg) month old Yorkshire pigs. The measurements were carried out on a Bruker 14.1T with an Avance III spectrometer, and completed within four hours from sacrifice.
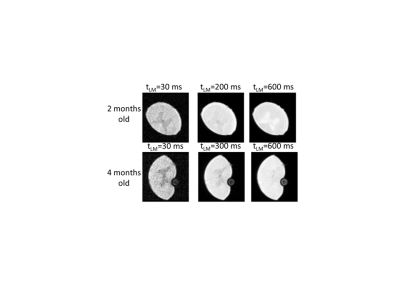
Results: In Fig. 1 we show the dependence of the images acquired by the SelSat pulse sequence on tLM. For tLM<400 ms the WM intensity is larger than that of the GM while for longer tLM values the image resembles the contrast observed in standard GE. The ratio, WM/GM, of average intensities of the white and grey matter varies with increasing exchange time, tLM, from 1.09 to 0.85 for the younger animal and from 1.26 to 0.85 for the older animal.
Discussion: In recent years it was shown in several studies1,3 that the fraction of protons residing in non-aqueous species can be extracted from on-water-resonance driven MT. In the current study we used SelSat (see material and methods and ref. 3). The set of images were analyzed in terms of two pools model1,3 under the fast and general exchange assumptions yielding small differences. Thus, in the current work we consider the results obtained using the fast exchange limit. The relevant expression is given by1:
Mzw(tLM)/Meqzw=F(1-exp(-tLM/texc))+(1-F) (1-exp(-tLM/T1)) (1)
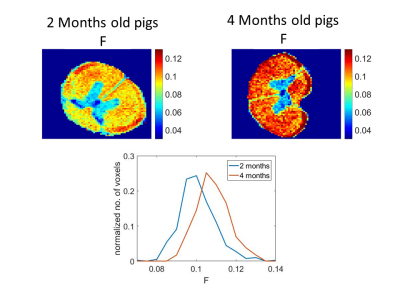
Where F is the fraction of protons residing in non-aqueous species, 1/texc= kwp+ kpw with kwp being the exchange rate between the water and non-aqueous species, and kpw is the reverse reaction, T1 is the water longitudinal relaxation, Meqzw is its equilibrium magnetization being proportional to the signal with tLM>>T1. The results are shown in Fig. 2. It is evident that the F is larger for the older animal. These changes in F with age are well demonstrated in the histogram (Fig. 2, bottom) where for the four months old pig the number voxels having F>0.11 is much greater than for the two months old. The opposite is true For F<0.1. This increase is consistent with previous reports about the increase of myelin content4,5 with age. The Figure also shows that F is larger in white matter as is expected due to its larger myelin content4,5.
Conclusion: MT driven by selective saturation of water enables the estimation of age-dependent changes in myelin content in spinal cord.
Acknowledgements
Acknowledgement: This study was supported by the US-Israel Binational Science Foundation grant number 2013253 and the IRP of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.References
1. Eliav U, Navon G. The role of magnetization transfer in the observed contrast in T1 weighted imaging under clinical setups. NMR Biomed. DOI: 10:1002/nbm.3792.
2. Stanisz GJ, Recojervic A, Bronskill MJ, Henkelman RM. Characterizing white matter with magnetization transfer and T2. Magn. Reson. Med. 1999;42:1128-1136.
3. Gochberg DF, Gore JC. Quantitative magnetization transfer imaging via selective inversion recovery with short repetition times. Magn. Reson. Med. 2007;57:437-441.
4. Wilhelm MJ, Ong HH, Wehrli SL, Li C, Tsai PH, Hackney DB, Wehrli FW. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc. Natl. Acad. Sci. 2012:109;9605-9610.
5. Sweasey D, Patterson DSP. Biphasic myelination and the fatty acid composition of cerebrosides and cholesterol esters in the developing central nervous system of the domestic pig. J. Neurochemistry 196:27;375-380.
Figures