5503
3D inhomogeneous magnetization transfer and rapid gradient echo (ihMTRAGE) imaging1Division of MR Research, Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 2CNRS, CRMBM, UMR 7339, Aix Marseille Universite, Marseille, France, 3GE Healthcare, Boston, MA, United States, 4GE Healthcare, Rochester, MN, United States, 5GE Healthcare, Menlo Park, CA, United States, 6GE Healthcare, Calgary, AB, Canada
Synopsis
The inhomogeneous magnetization transfer (ihMT) technique provides a myelin-sensitive signal and has been applied for 3D acquisition in the steady-state. Sequences applied in a segmented fashion, following some magnetization preparation, provide an advantage of allowing insertion of additional modules, e.g. motion correction. An ihMT acquisition in the style of the magnetization-prepared rapid gradient-echo sequence was designed based on considerations of safety, hardware and optimizing the ihMT signal. Whole brain 3D ihMT data with 2.4mm isotropic resolution was achieved in 6-7mins. IhMT ratios between 15-20% were measured in white matter areas, and were not significantly modified by inclusion of a prospective motion correction module.
Introduction
Inhomogeneous magnetization transfer (ihMT) provides a contrast sensitive to myelinated structures1. Its signal is correlated with myelin content as measured by fluorescence microscopy2, and other MRI techniques that are surrogate measures of myelin3. State-of-the-art 3D ihMT relies on RF pulses applied off-resonance and readout(s) combined and repeated to achieve a steady-state signal4. A preparatory module is used to prepare the magnetization and allows rapid 3D acquisition in a segmented fashion. Such magnetization preparation sequences allow the amount of k-space sampled to be controlled depending on motion or expected changes in signal. These sequences can also be adapted to include prospective motion (PROMO) compensation or other modules5. Recent developments show the maximum ihMT signal available can be greatly enhanced with reduced RF duty cycles4. Whilst this allows for lower SAR sequences, the maximum achievable pulse amplitude limits the ihMT signal. The goal of this work is to demonstrate an ihMT prepared 3D gradient echo sequence in the style of the magnetization-prepared rapid gradient-echo (MPRAGE) acquisition.Methods
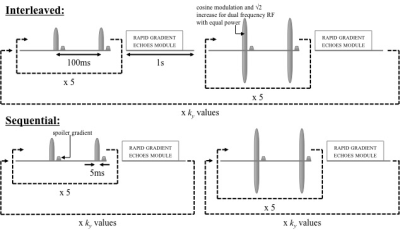
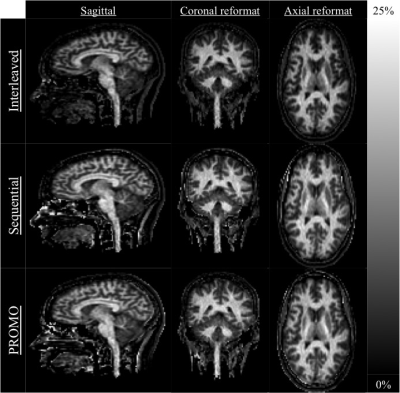
Simulations were conducted for white matter (WM) and grey matter (GM) tissues using numerical solution of the differential equations of the two-pool model of MT (ode45, Matlab, MathWorks). Single frequency off-resonance irradiation was simulated by inclusion of a dipolar reservoir6. Values for rate and other quantitative MT parameters were taken from prior literature at 3T7-8. Imaging was achieved with a series of MT pulses applied at ±7kHz (B1,peak=150mG from scanner limitations) followed by rapid gradient-echoes (RAGEs), based on simulation results, repeated for a given k-space volume. RAGEs were simulated by multiplication of the free-pool longitudinal magnetization by the cosine of the flip angle (FA), assuming perfect spoiling. Since ihMT involves separate single and dual frequency RF preparations, an interleaved strategy was compared to acquisition of 3D datasets with one preparation type in a sequential manner. Figure 1 illustrates the strategies employed on a 3T clinical scanner (Discovery MR750, GE Healthcare) for whole-brain ihMT. The repetition time between cycles of the MT preparation plus RAGEs, TRRAGE was increased from 2 to 2.5s to include the PROMO module. Whole brain ihMTRAGE data were acquired in healthy volunteers, after informed consent, using a 32-channel head coil. IhMT was calculated as twice the difference between the signals following single and dual frequency RF, and ihMTR by dividing ihMT by the signal following zero power preparation.Results
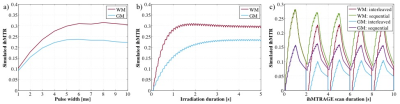
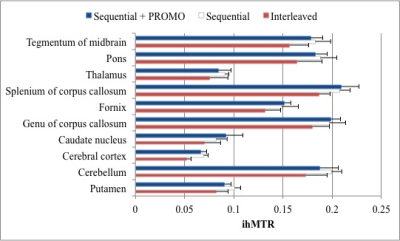
Taking a fixed, low duty cycle of 5% for the MT preparation, the simulated ihMT signal after 4s of irradiation for different pulse widths was maximal for 5ms RF in GM and 95% of the maximum in WM (Fig. 2a). Simulation of the ihMT following cycles of 5ms MT pulses repeated every 100ms shows a steady-state is reached after 3s (Fig. 2b). However, the ihMT is within 8 and 35% of the steady-state value, for WM and GM tissues respectively, after a more reasonable 1s preparation. Although an interleaved acquisition might reduce the interference of motion between the different preparations, a reduction in ihMTR was observed in simulations and acquired data (Figs. 2c-4). A significant difference (p<0.01 from paired one-tail t-tests) between the ihMTRs from an interleaved and sequential implementation was measured in: putamen; cerebellum; cerebral cortex; splenium of corpus callosum; pons; and, midbrain regions of interest (Fig. 4). No significant change in ihMTRs was found from inclusion of PROMO.Discussion
The results from simulations of the ihMTRAGE sequence, accounting for scanner limitation information, allowed MPRAGE like acquisition for whole brain ihMT. Lower ihMTRs from an interleaved acquisition were close to that expected from simulation using a low duty cycle preparation. IhMTR values lower than simulations predict were likely the result of ihMT signal decay during acquisition and/or inaccurate parameter values (Figs. 2,4). Simulation of RAGEs following MT preparation showed decreasing ihMT with increasing FA and acquisition time. This result supported a center-out k-space acquisition and minimizing FA, but requires further consideration of signal-to-noise effects. Corpus callosum ihMTRs were greater than those reported for a 3D steady-state gradient-echo implementation4, likely due to the relatively concentrated energy deposition scheme (B1,RMS~30mG over the preparation) and dual frequency preparation by cosine modulation9. Simulations provide a framework for optimization of ihMTRAGE based on tissue parameters and other considerations, including duty cycle, the signal-to-noise for variable FAs and different TRs of the RAGEs.Conclusion
A 3D ihMT sequence based on MPRAGE was implemented for acquisition of 2.4mm isotropic data of the brain in scan times of 6-7mins. Simulations of the sequence successfully anticipated the reduced ihMTR values observed in an interleaved relative to sequential acquisition and can guide further optimization of ihMTRAGE.Acknowledgements
No acknowledgement found.References
1. Varma G, Duhamel G, de Bazelaire C, et al. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med. 2015;73(2):614-622.
2. Prevost V, Girard O, Cayre M, et al. Validation of inhomogeneous magnetization transfer (ihMT) as a myelin biomarker. Proc. ISMRM 2017:4549.
3. Geeraert BL, Lebel RM, Mah AC, et al. A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. NeuroImage. 2017;doi:10.10156/j.neuroimage.2017.09.019.
4. Mchinda S, Varma G, Prevost VH, et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn Reson Med. 2017;doi:10.1002/mrm.26907.
5. White N, Roddey C, Shankaranarayanan A, et al. PROMO: Real-time prospective motion correction in MRI using image-based tracking. Magn Reson Med. 2010;63(1):91-105.
6. Varma G, Girard OM, Prevost VH, et al. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J Magn Reson. 2015;260:67-76.
7. Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54(3):507-512.
8. Varma G, Girard OM, Prevost VH, et al. In vivo measurement of a new source of contrast, the dipolar relaxation time, T1D, using a modified inhomogeneous magnetization transfer (ihMT) sequence. Magn Reson Med. 2017;78(4):1362-1372.
9. Prevost VH, Girard OM, Mchinda S, et al. Optimization of inhomogeneous magnetization transfer (ihMT) MRI contrast for preclinical studies using dipolar relaxation time (T1D) filtering. 2017;doi:10.1002/nbm.3706.
Figures