5502
3D Steady-State Inhomogeneous Magnetization Transfer (ihMT) Gradient Echo Sequence for Spinal Cord Imaging at 3T1Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 2Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 3Radiology, Division of MR Research, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 4Philips Healthcare, Gainesville, FL, United States
Synopsis
Inhomogeneous magnetization transfer (ihMT) is an enhanced magnetization transfer method, which is sensitive to dipolar couplings in white matter. Current spinal cord applications of ihMT use long saturation followed by single-slice acquisitions. As an alternative to this implementation, in our study we implemented and optimized a pulsed ihMT-prepared 3D gradient echo sequence for a larger coverage of spinal cord. The in vivo ihMT results from cervical spinal cord demonstrate potential for future applications of the ihMT in the spinal cord.
Introduction
Inhomogeneous magnetization transfer (ihMT) is a new contrast method, which is sensitive to the dipolar couplings from long lipid chains and is suggested to be a new marker of myelin1-5. In vivo applications of ihMT have recently been extended from the human brain to the cervical spinal cord6-10. The spinal cord applications showed disease-related changes in the cervical spinal cord regions of patients with amyotrophic lateral sclerosis8 and multiple sclerosis9. All these applications employed a long off-resonance frequency saturation (~500 ms) followed by a cardiac triggered single-slice 2D spin-echo acquisition. This acquisition scheme requires a very long acquisition time (e.g. 8:00 min per slice) and may not be practical for routine clinical applications. Recently, whole-brain ihMT imaging was demonstrated using a 3D steady-state ihMT gradient echo acquisition11. This method provides larger coverage within shorter acquisition times as well as a more efficient saturation via the use of steady-state saturation, and can be also beneficial for spinal cord applications. In this study, we implemented, optimized and demonstrated for the first time a 3D steady-state ihMT gradient echo (GRE) sequence for a large coverage of the cervical spine. Furthermore, we investigated strategies to compensate for physiological motion affecting the spinal cord and explored replacing standard GRE readout with EPI.Methods
All experiments were performed on a 3T Ingenia Philips MRI scanner (Philips Healthcare, Best, The Netherlands) equipped with a dual-transmit body coil and a 16-channel neurovascular receive head-coil. Three healthy volunteers (one female, age: 30 ± 5 years) participated in the study with the IRB approval and written informed consent. 3D steady-state ihMT images were acquired from the cervical spine (C3-C7).
Acquisition parameters: IhMT preparation was applied at each TR and employed 6 Hann-shaped RF pulses (pulse duration = 0.9 ms, flip angle = 90°, RF phase cycling = 117°, Δf = ±7000 Hz, peak B1 amplitude of 13 mT and B1rms of 4.98 mT over the entire ihMT preparation duration of 13.8 ms), as shown in Figure 1. A reverse linear k-space acquisition scheme with partial Fourier encoding was used to achieve saturation steady state by the time of the acquisition of the center of the k-space. A reference image (M0) and 4 separate ihMT images (frequency offsets: +7 kHz (MT+), -7 kHz (MT-), alternating ±7 kHz (MT+-) and alternating -+7kHz (MT-+)) were obtained. The imaging parameters for the 3D steady-state ihMT gradient echo acquisitions were as follows: excitation flip angle (α)=10°, TR/TE =90/1.77ms, FOV=160x160x90mm3, resolution=1x1mm2, slice thickness=10mm and total acquisition time of 4 min 36 s. To compensate for pulsatile motion, two strategies were also tested: flow compensation and cardiac gating through a peripheral pulse triggering. Cardiac gating resulted in an increase in the scan time, up to 8.5 minutes. To reduce the scan time, segmented acquisitions using echo-planar imaging (EPI) read-outs were acquired (TR/TE =90/3.8 ms, EPI factor = 7, NSA=3, total acquisition time = 6 min 17 s) with the same saturation scheme, resolution and FOV.
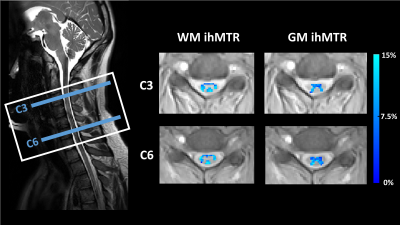
Post-processing: For motion correction, all ihMT prepared images were co-registered to the reference (M0) image using the Spinal Cord Toolbox12 (SCT) (Figure 2). SCT was then used to segment gray matter (GM) and white matter (WM) in each slice13 (Figure 2). A customized MATLAB code was used to calculate the ihMT ratio according to the following equation: ihMTR = (MT+ +MT- - MT+- - MT-+)/ M0. The gray and white matter masks were used to calculate the mean ihMTR from white and gray matter in each slice.
Results and Discussion
In Figure 3, ihMTR values obtained using the 3D steady-state ihMT gradient echo acquisition are shown for two different levels of the cervical spinal cord. Table 1 shows mean ihMTR values from the standard non-gated GRE and the cardiac gated EPI read-out implementations. No substantial difference was observed between the standard 3D steady-state GRE and gated EPI acquisitions. Thus, EPI read-out can be used to speed up the acquisition time in cardiac-gated acquisitions without a compromise in the ihMT effect. The mean spinal cord ihMTR values found in this study are higher than those reported using single-slice spin-echo based ihMT sequences7-10. But the values are consistent with the steady-state based sensitivity enhancement of the brain ihMT values in the previously proposed gradient echo implementation of the whole brain11. Flow compensation resulted in artifactual ihMTR maps (data not shown).Conclusion
A pulsed ihMT-prepared 3D SPGR sequence is feasible in the human cervical spinal cord and is promising for future spinal cord applications. Work in progress includes further strategies for correction of respiratory and pulsatile motion.Acknowledgements
The authors would like to thank Kelli Key and Trevor Wigal for helping with subject recruitment and MRI scans.References
1. Varma G, Duhamel G, de Bazelaire C, Alsop DC. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med 2015;73(2):614-22.
2. Girard OM, Prevost VH, Varma G, Cozzone PJ, Alsop DC, Duhamel G. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn Reson Med 2015;73(6):2111-21.
3. Swanson SD, Malyarenko DI, Fabiilli ML, Welsh RC, Nielsen JF, Srinivasan A. Molecular, dynamic, and structural origin of inhomogeneous magnetization transfer in lipid membranes. Magn Reson Med 2016.
4. Varma G, Girard OM, Prevost VH, Grant AK, Duhamel G, Alsop DC. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. J Magn Reson 2015;260:67-76.
5. Manning AP, Chang KL, MacKay AL, Michal CA. The physical mechanism of "inhomogeneous" magnetization transfer MRI. J Magn Reson 2017;274:125-136
6. Girard OM, et al. Magnetization transfer from inhomogeneously broadened lines (ihMT): improved imaging strategy for spinal cord applications Magn. Reson. Med. 2016. DOI:10.1002/mrm.26134
7. Taso M, Girard OM, Duhamel G, Le Troter A, Feiweier T, Guye M, Ranjeva JP, Callot V. Tract-specific and age-related variations of the spinal cord microstructure: a multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed 2016;29(6):817-32.
8. Rasoanandrianina H, Grapperon AM, Taso M, Girard OM, Duhamel G, Guye M, Ranjeva JP, Attarian S, Verschueren A, Callot V. Region-specific impairment of the cervical spinal cord (SC) in amyotrophic lateral sclerosis: A preliminary study using SC templates and quantitative MRI (diffusion tensor imaging/inhomogeneous magnetization transfer). NMR Biomed 2017
9. Rasoanandrianina et al. in Proceedings of ISMRM 2017: p419.
10. Rasoanandrianina et al. in Proceedings of ISMRM 2017: p912.
11. McHinda S, Varma G, Prevost VH, et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn Reson Med 2017 Sep 23. doi: 10.1002/mrm.26907.
12. De Leener B, Levy S, Dupont SM, Fonov VS, Stikov, N, Collins DL, Callot V, Cohen-Adad J, Sct: Spinal cord toolbox, an open-source software for processing spinal cord mri data. NeuroImage 2017; 145:24-43.
13. Dupont SM, De Leener B, Taso, M, Le Troter A, Stikov N, Callot V, Cohen-Adad J, Fully-integrated framework for the segmentation and registration of the spinal cord white and gray matter. NeuroImage 2017; 50: 358-372.
Figures