5485
Serotonergic Excitability and Functional Connectivity in Acute, Chronic, and Withdrawal Phases of Antidepressant Treatment1Institute for Biomedical Engineering, ETH and University of Zurich, Zürich, Switzerland, 2Preclinical Laboratory for Translational Research into A ective Disorders, DPPP, Psychiatric Hospital, University of Zurich, Zürich, Switzerland
Synopsis
The serotonergic system is characterized by high centrality and is implicated in affective disorders and their treatment. During longitudinal antidepressant (fluoxetine) administration in mice, we optogenetically stimulate the ascending serotonergic system, and infer brain activity via fMRI. We resolve serotonergic excitability and functional connectivity at 5 time points and show that a network representation is needed to adequately model the data. For acute and chronic sessions we document significantly increased serotonergic excitability, and distinctly multivariate effects on serotonergic transmission. Finally, we find a full brain function recovery to baseline after fluoxetine withdrawal.
Introduction
The serotonergic system lies at the crossroads of basic and applied research. It represents a highly connected brain node which is known to affect emotion and behavior. The study of this system presents an excellent opportunity to advance brain network analysis, and bring resulting insights to bear on high human impact phenomena. This highly evolutionarily conserved1 system is ideal for translational study in mice, where higher causal certainty can be attained by driving rather than just observing serotonergic function.
Selective Serotonin Reuptake Inhibitors (SSRIs) are the foremost antidepressant drug class, and also find significant use in treating anxiety, phobia, panic, and obsessive-compulsive disorders2. SSRIs are known for their chronic mechanism of action, which is not explained by a unified framework3. They are also known for psychophysiological withdrawal effects, showing a temporal profile similar to the half-life of each substance4.
We employ diverse fMRI analysis tools to establish a serotonergic network effect model for the acute, chronic, and withdrawal stages of SSRI treatment, in order to further our mechanistic understanding of the serotonergic system - and our capacity to predictably manipulate it.
Methods
Dorsal Raphe (DR) serotonergic cells were targeted for optogenetic stimulation by injecting Cre-conditional channelrhodopsin-expressing adeno-associated viruses into mice expressing Cre under the ePet promoter5. A chronic optic fiber implant provided access to the DR. Mice were imaged and stimulated according to the same protocol in 2-week intervals for 5 sessions: before, during acute, chronic (2x), and withdrawal from an SSRI treatment (fluoxetine). Acute treatment was intravenous, chronic treatment via drinking water. A control group was treated with vehicle.
Measurements were performed under isoflurane and medetomidine anesthesia6. Laser stimulation at 30mW and 488nm was delivered via a microsecond-precision controller7. Echoplanar imaging was performed with continuous coronal coverage at a 225µm in-plane and 450µm axial resolution (TE/TR=5.9/1000ms). Cerebral Blood Volume contrast was obtained via iron oxide nanoparticles (Endorem, Laboratoire Guebet SA, France).
Data preprocessing and analysis (via SAMRI8) encompassed quality controlled registration, supervised smoothing, mass univariate linear modelling, analysis of variance (ANOVA) in both ROI and voxel space, multivariate pattern analysis, and seed-based functional connectivity estimation.
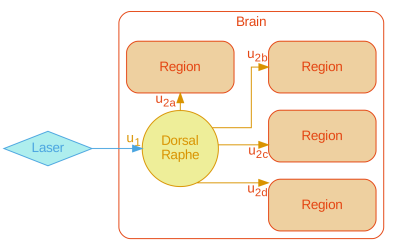
A graph representation of the serotonergic system was formulated (Figure1) to provide cell biological context for the aforementioned analysis modes.
Results
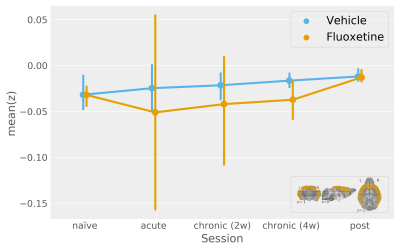
A multivariate pattern reflecting the bimodal response from the naïve condition finds only tentative fluoxetine treatment effects for a uniformly scaling serotonergic network (Figure2).
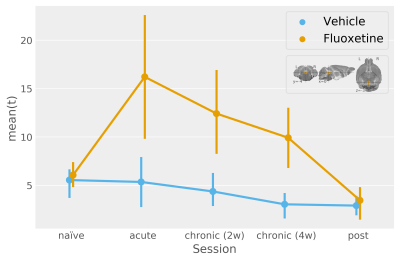
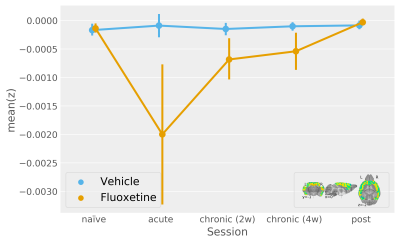
In contrast, by resolving the serotonergic network in accordance with Figure1, we find a highly significant effect of both acute and chronic fluoxetine treatment on serotonergic excitability (Figure3). Seed-based functional connectivity analysis, however, reveals only a visual trend towards increased transmission from DR to the cortex during acute and chronic fluoxetine administration (Figure4).
We refine our DR-to-cortex functional connectivity analysis based on a multivariate ANOVA map (with Z-scores reflecting how each voxel is deviating between fluoxetine treatment sessions). The timecourse thus obtained shows a significant fluoxetine treatment effect (Figure5) and it is is notable that this evaluation cannot be reproduced by an ROI (i.e. thresholding of the Z-score map, data not shown).
In all comparisons there is no significant effect (on either serotonergic excitability or transmission) of longitudinal treatment in the control group. We observe low variation in the group, and no group effect outside the treatment bracket (including during fluoxetine withdrawal). These features speak to the reliability of our data and the stability of the read-out in the absence of a pharmacological challenge.
Discussion
Our results show that uniform (even if multivariate) modelling of the serotonergic system during longitudinal fluoxetine treatment cannot adequately fit the salient features of the data, and that network representation is a necessity in serotonergic modelling.
The increase in serotonergic excitability for acute fluoxetine treatment runs contrary to canonical SSRI mechanistc explenations3, which postulate that DR activity should be reduced via autoinhibition for acute SSRI treatments.
The capacity of a signed salience-weighted pattern (but not a ROI) to isolate a significant treatment effect indicates that serotonergic transmission modulation by fluoxetine is multivariate. The multivariate pattern itself follows a loose topographic organization, with fluoxetine-mediated transmission increases deeper and transmission decreases more superficially in the cortex. This tentative interpretation signals the need to more closely resolve sertotonergic functional connectivity - potentially requiring higher statistical power and further method refinement.
Remarkably, the effects we document during fluoxetine treatment cease at two weeks after withdrawal, and are not replaced by (mal)adaptive reorganizations. This indicates that fluoxetine may not be able to effect long-term plasticity in the healthy mouse brain, or at the very least not in a fashion accessible to our current methods.
Acknowledgements
No acknowledgement found.References
1. Turlejski K. Evolutionary ancient roles of serotonin: long-lasting regulation of activity and development. Acta Neurobiol Exp (Wars). 1996;56(2):619-36.
2. D. S. Baldwin. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendationsfrom the british associationfor psychopharmacology. Journal of Psychopharmacology, 19(6):567–596, nov 2005. doi:10.1177/0269881105059253.
3. David J.Nutt, Sam Forshall, Caroline Bell, Ann Rich, John Sandford, Jon Nash, Spilios Argyropoulos. Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. European NeuropsychopharmacologyVolume 9, Supplement 3, July 1999, Pages S81-S86 doi:10.1016/S0924-977X(99)00030-9
4. Thibault Renoir. Selective Serotonin Reuptake Inhibitor Antidepressant Treatment Discontinuation Syndrome: A Review of the Clinical Evidence and the Possible Mechanisms Involved. Front Pharmacol. 2013; 4: 45.Published online 2013 Apr 16. doi: 10.3389/fphar.2013.00045
5. M. M. Scott, C. J. Wylie, J. K. Lerch, R. Murphy, K. Lobur, S. Herlitze, W. Jiang, R. A. Conlon, B. W. Strowbridge,and E. S. Deneris. A genetic approachto access serotonin neurons for in vivo and in vitro studies. Proceedings ofthe National Academy of Sciences, 102(45):16472–16477, oct 2005. doi:10.1073/pnas.0504510102. URL http://dx.doi.org/10.1073/pnas.0504510102.
6. Joanes Grandjean, Aileen Schroeter, Imene Batata, and Markus Rudin. Optimization of anesthesia protocol for resting-state fmri in mice based on differential effects of anesthetics onfunctional connectivity patterns.NeuroImage, 102 Pt 2:838–847, November 2014.
7. Aymanns, Florian, Ioanas, Horea-Ioan, & Rudin, Markus. (2017). Contrast Optimized Stimulus generator (COSgen). European Conference on Python in Science (EuroSciPy), Erlangen, Germany, 28-31 August 2017 (Session X ) DOI: doi.org/10.5281/zenodo.1000450
8. Horea-Ioan Ioanas, Markus Marks, Dominik Schmidt, Florian Aymanns, & Markus Rudin. (2017, November 8). SAMRI - Small Animal Magnetic Resonance Imaging (Version 0.1). Git: git@github.com:IBT-FMI/SAMRI.git DOI: doi.org/10.5281/zenodo.1044033
Figures
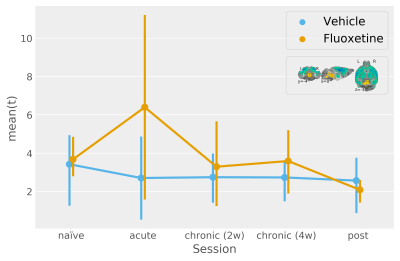
Mean product of participant t-statistic maps and the unthresholded naïve condition t-statistic map (the legend shows the naïve condition t-statistic map thresholded at +/-3 for more clarity with respect to the template). This procedure models multivariate activity under the assumption of uniform system scaling. F-statistic (modelling deviation within the "sessions by fluoxetine treatment" interaction levels jointly with an absence of a net effect for vehicle treatment and "sessions by vehicle treatment" interactions) approaches significance for this multivariate pattern [F(5, 26)=2.06, p=0.1].