5464
A comparison of distortion correction methods for EPI fMRI applied across three 7T platforms1Cardiff University Brain Research Imaging Centre, School of Psychology, Cardiff University, Cardiff, United Kingdom, 2Sir Peter Mansfield Imaging Centre, School of Physics and Astronomy, University of Nottingham, Nottingham, United Kingdom, 3Wellcome Centre for Integrative Neuroimaging (FMRIB), Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 4Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom, 5Institute of Neuroscience & Psychology, University of Glasgow, Glasgow, United Kingdom
Synopsis
In preparation for a wider multi-site ‘travelling heads’ 7T fMRI study, we compare the performance of EPI distortion correction techniques for fMRI data across four sites, using three different 7T platforms. Specifically, we compare B0-map and phase-encoding reversal methods, applied to both task and resting state fMRI data.
Introduction
To make best use of the improved resolution of fMRI at 7T,1 correction of the susceptibility-induced distortions in EPI data is often employed. In this work, we evaluate two widely used distortion correction techniques, specifically phase-encoding reversal2 and fieldmap3 based methods, and assess the performance of each method on different 7T scanners. The motivation for this work is to generate a ‘travelling head’ fMRI dataset acquired using a matched protocol across three 7T platforms. This dataset was acquired to allow multiple options for distortion correction to assess the optimal method across scanner platforms.Methods
This study was approved by local ethics review boards at each site and prior informed written consent was obtained from participants. Data were acquired in 3 healthy male participants (31±4 years) at four sites, using three different 7 Tesla whole-body MRI systems (one Philips Achieva, two Siemens Magnetom and one Siemens Terra scanners). The same model volume transmit, 32-channel receive head coil (Nova Medical) was used at each site and QA data on the day of scanning confirmed similar performance.
Both resting state (1s TR, multi-band 3) and motor-visual task (2s TR, multi-band 2) gradient echo (GE) EPI fMRI datasets4 were acquired (25ms TE, 1.5mm isotropic, echo spacing 0.68/0.75ms Siemens/Philips). For distortion correction, two separate reference scans were acquired (each 3 volumes), one with matched phase-encode direction, the other with reversed phase-encode direction. This was repeated for both GE and spin echo (SE) EPI reference scans (acquisition matrix, echo spacing, bandwidth matched to fMRI). Dual-echo GE B0-maps (1ms ΔTE) were also acquired at 2mm resolution. T2*-weighted anatomical (FLASH) data were acquired (0.75x0.75x1.5mm, TE 10ms, TR 1100ms) for image registration. All reference scans were acquired separately for, and immediately prior to, resting state and task fMRI datasets to match acquisition geometries.
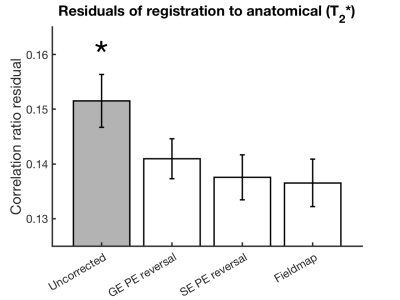
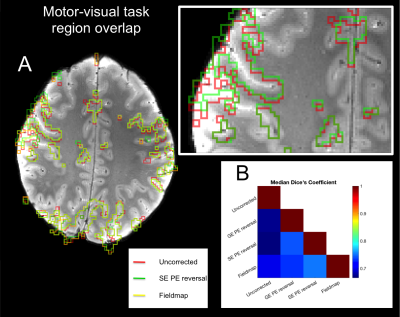
With the exception of the different B0-map processing between Siemens and Philips (output data as phase difference and field map in Hz, respectively), a single analysis pipeline worked across all four sites. Phase-encoding reversal distortion correction was performed using FSL TOPUP,2 whilst the field-map method used FSL FUGUE.5 Quality of the distortion corrections were assessed through the residuals of rigid-body registration (correlation ratio cost function value) to the T2*-weighted anatomical and through assessing how the task-activation (FSL FEAT5) and resting state network (FSL MELODIC5) regions aligned to sulci in the T2*-weighted anatomical. Dice’s coefficient was used to measure relative spatial overlap between functional regions in image data generated using different distortion correction methods.
Results
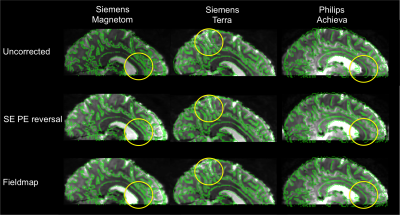
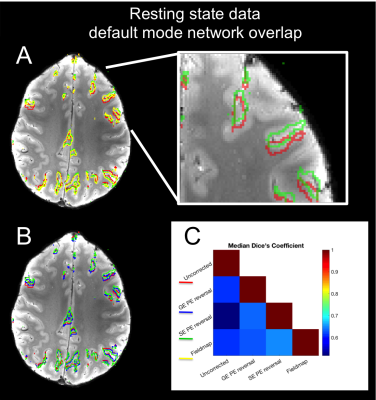
The performance of SE phase-encoding reversal and fieldmap distortion correction methods was similar across all three platforms (Figure 1). Rigid body registration residuals (Figure 2) show that all distortion-corrected datasets have better alignment (lower residuals, p<0.001) to the T2*-weighted anatomical compared with the uncorrected data. This is also shown in the overlap of task-activation regions between the SE phase-encoding reversal and fieldmap methods, with better alignment to the anatomical sulci than in the uncorrected data (Figure 3). Whilst SE phase-encoding reversal and fieldmap methods performed similarly (Figure1/4A), there is a trend for the GE phase-encoding reversal method to perform less well (Figure 1/4B), as shown with a better alignment of default mode network regions to sulci in the T2*-weighted anatomical for SE phase-encoding reversal and fieldmap methods, compared with GE phase-encoding reversal and the uncorrected dataset. Note, the default mode network was chosen for its reproducibility across repeated experiments across sites.Discussion
Despite differences in the distortions across subjects and sites (likely due to head position differences), the distortion correction methods perform similarly across all three 7T platforms studied. The phase-encoding reversal method was designed for SE EPI data. The SE phase-encoding reversal reference scans allowed comparable distortion correction of the GE fMRI datasets to the fieldmap method. This method was easier to implement than the fieldmap method, in terms of image registration and the recording of acquisition-specific parameters required for implementation. The GE phase-encoding reversal method performed less well, which is likely due to the GE EPI acquisition violating the assumptions of the method, due to signal drop-off around field offsets. Despite this, whilst not performing as well as the other methods, it was still better than no correction. This work focuses on the performance of distortion correction methods for a specific acquisition protocol, specifically matched across multiple 7T platforms. A comprehensive comparison of the performance of distortion correction methods more generally would require exploration across the range of likely fMRI acquisition protocols.Conclusion
Both fieldmap and SE phase-encoding reversal distortion correction methods behave similarly across multiple 7T sites.Acknowledgements
We acknowledge the UK Medical Research Council for funding support (MR/N008537/1). The Siemens sites used EPI multiband sequence software provided by University of Minnesota Center for Magnetic Resonance Research.References
1. Shmuel A, Yacoub E, Chaimow D et al. Spatio-temporal point-spread function of fMRI signal in human gray matter at 7 Tesla. NeuroImage 2007;35:539-552.
2. Andersson J, Skare S and Ashburner B. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003;20:870-888.
3. Jezzard P and Balaban R. Correction for geometric distortion in echo planar images from B0 field variations. Magn. Reson. Med. 1995;34:65–73.
4. Moeller S, Yacoub E, Olman C et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63:1144-1153.
5. Smith S, Jenkinson M, Woolrich, M et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208-219.
Figures