5454
THE ANGULAR DEPENDENCE OF THE GRADIENT ECHO AND SPIN ECHO BOLD SIGNAL INDUCED BY CORTICAL MICRO- AND MACRO- VASCULATURE1Department of High-Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Graduate Training Centre of Neuroscience, University of Tuebingen, Tuebingen, Germany, 3Department of Physics, University of California at San Diego, San Diego, CA, United States, 4Section of Neurobiology, University of California, San Diego, CA, United States, 5Department of Biomedical Magnetic Resonance, University of Tuebingen, Tuebingen, Germany
Synopsis
In recent investigations, the angular dependence of the BOLD signal with respect to the main magnetic field has been shown by Monte Carlo simulations and experimental approaches. This orientation dependence is often attributed to the contribution of large vessels (cortical surface and penetrating arteries/veins). However, the ability to resolve cortical layers and columns depends ultimately on the contribution of the MR signal generated by the capillary bed. In this work, we studied the MR signal attenuation generated by a vascular network model that was acquired from the parietal cortex of mice using a two-photon laser imaging techniques. We separately investigated the impact of macrovessels (>5 µm in diameter) and microvessels (< 5 µm in diameter) on the BOLD effect.
INTRODUCTION
In recent investigations, the angular dependence of the BOLD signal with respect to the main magnetic field has been studied by means of Monte Carlo simulations [1,2] and experimental approaches [3]. Gagnon (2015) and Fracasso (2017) showed a cos2(θ) dependence in the BOLD signal with respect to the main magnetic field. For gradient echoes (GE) this angular dependence of the signal change is attributed to the contribution of larger vessels (cortical surface and penetrating arteries/veins). For spin echoes (SE) the macrovascular contribution is reduced but is not completely eliminated. The ability to resolve cortical layers and columns depends ultimately on the contribution of signals from the capillary bed. Taking advantage of the high spatial resolution gained at ultrahigh magnetic fields and the increased contribution of the capillary components, in theory, it could be possible to extract information from different cortical depths. To date, it is not possible to image mesoscopic spatial scales with MRI. However, we can simulate the MR signal behavior on realistic models and analyze the signal intensity and/or signal changes under certain conditions and parameters. In this work, we simulated different oxygenation level scenarios in a vascular network model from the parietal cortex of mice acquired using a two-photon laser imaging techniques [5]. We analyzed the MR signal attenuation that, independently, macrovessels (> 5 µm in diameter) and microvessels (<5 µm in diameter) produce under active and resting state conditions (deoxygenated/oxygenated state).METHODS
To understand the MR signal contributions, it is necessary to analyze the features of the neurovascular model. The orientation of the vasculature is fundamental. Two different analysis of orientation distribution were performed. First, a polar density distribution, where the polar radius represents the radius of the vessel and the polar angle shows the vessel orientation dependence with respect to a vector normal to the cortical surface. Second, a density distribution represents the probability of the orientation. GE and SE sequences were simulated on separated macrovascular and microvascular compartments. The simulation was implemented in Matlab 2016a and C/C++ with 128 cores in parallel computation. We follow the same procedure as Weisskof and Boxerman [6,7] to obtain the MR signal decay. Briefly, this method stores the phase acquired for a certain echo time (TE) per spin, and the signal attenuation can be calculated as the average of all spins. Diffusion motion was simulated with random walkers (1µm2/ms) . 3X105 spins were simulated. Active and resting states were modeled with a certain oxygenation level. The BOLD signal changes take into account extravascular and intravascular contributions. T1/T2* for GE: Act:2200/8ms, Rest:2200/4ms; T1/T2 for SE: Act:2200/20ms, Rest:2200/12ms; T1/T2 for extravascular: 2200/40ms. All simulations were performed under a main magnetic field of 9.4T. To obtain the %BOLD signal change for each orientation ((act – rest)/act)X100 was calculated.
RESULTS
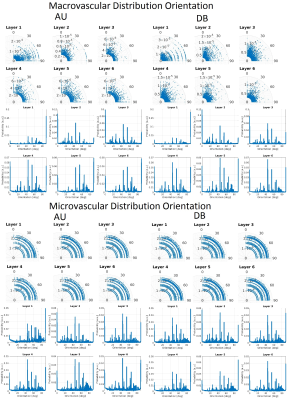
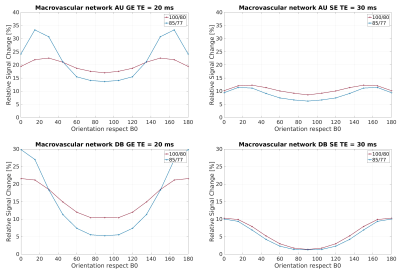
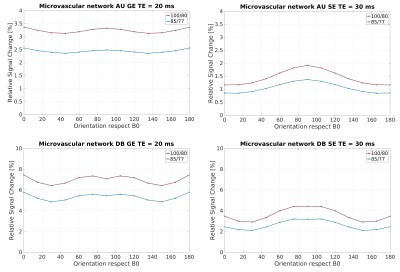
In Figure 1, the density distribution is presented as a polar distribution of the orientation of the vessels with reference to a vector normal to the cortical surface together with the vessel’s radius for all orientations, moreover, a density distribution of the orientations. In Figure 2 and Figure 3, we show the macrovascular and microvascular angular dependence of the BOLD signal change from the average of 6 equidistant layers under two different oxygenation level conditions: first, a 100% active state vs. 80% resting state oxygenation; and second, an 85% / 77% oxygenation level.
DISCUSSION/ CONCLUSION
The vascular models show different topography in their architecture. In Figure 1, the macrovessels tend to form clusters in some vessel size regions. For instance, in layer 1 the contribution of the cortical surface arteries/veins can be observed as a cluster around 90°, moreover, from layer 2 to 6, the penetrating vessels present a parallel contribution around 0° with respect the reference vector. In contrast, the orientation distribution of microvascular vessels is close to random. The sensitivity of GE to larger vessels is higher than that of SE [6,7]. This phenomenon is apparent in Figure 2. The macrovessels in GE exhibit a larger BOLD signal change compared with the SE BOLD signal change. Furthermore, SE BOLD is more sensitive to oxygenation level. The angular dependence in SE is largerly reduced compared to GE, but not eliminated. These results are in line with previous reports [1,2,3]. For microvessels, the orientation dependence of the BOLD signal has an inverted behavior compared to the angular dependence of the macrovasculature. It is worth noting that the signal changes induced by the microvessels are relatively small and the angular dependence tend to be qualitatively similar for GE and SE. Remarkably, although microvessels are basically randomly oriented, their BOLD signal shows a larger signal intensity at 90°, where the magnetic field is parallel to them.Acknowledgements
No acknowledgement found.References
1. Gagnon L. et al, Quantifying the microvascular origin of BOLD fMRI from first principles with two-photon microscopy and an oxygen-sensitive nanoprobe, J Neurosci (2015) 35:3663-3675
2. Baez-Yanez MG et al, The impact of vessel size, orientation and intravascular contribution on the neurovascular fingerprint of BOLD bSSFP fMRI, NeuroImage (2017) 163:13-23
3. Fracasso A et al, Laminar imaging of positive and negative BOLD in human visual cortex at 7T, Neuroimage (2017) in press
4. Goense J et al. fMRI at high spatial resolution: implications for BOLD models. Front Comput Neurosci (2016) 10:66
5. Blinder P et al, The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nature Neurosci (2013) 16:889-897
6. Weisskoff et al, Microscopic Susceptibility Variation and Transverse Relaxation: Theory and Experiment, MRM (1994) 31:601-610
7. Boxerman J et al. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med (1995) 34:555-566
Figures