5452
Real-time processing of image and trajectory data: Application to single-shot time series1ETH Zurich, Zurich, Switzerland
Synopsis
The steady increase in data volume is a significant problem in fMRI time series. Recently proposed signal processing architectures based on digital hardware enable data reduction in real-time before the data are stored. We demonstrate that a high degree of data savings can be achieved with few simple operations. Coil compression and field probe data processing help breaking the data bottleneck in fMRI time-series.
Introduction
Single-shot time series rely on fast acquisition of high quality images. Continuing advances in scanner hardware and imaging methods push the boundaries on achievable SNR and acquisition speed. Especially the advent of parallel imaging1,2,3 has enabled higher spatial resolution and more robust scans with a better time resolution due to shorter readouts. More recent developments go even towards simultaneous acquisition of multiple slices utilizing multiband excitation.4 Those techniques rely on large receive arrays with up to 32 channels now already clinical standard with a trend to more.5,6,7 On the other hand, this development has led to a massive increase in data to be transferred, stored, reconstructed and analyzed. The trend further accelerated as auxiliary sensor systems8,9,10,11,12,13,14 pave their way into MRI, which introduce advanced signal models in image reconstruction. Especially optical motion tracking cameras11,13 and field monitoring10 generate additional data at bandwidths even beyond the imaging data. This is challenging, as they require a different processing chain. Recently, a real time processing platform15,16 has been presented providing high-throughput digital fiber-optical inputs for modern receiver and sensor modules with in-field operation17. In this work, we apply a reconfigurable signal processing architecture based on a system-on-chip (SoC) for single-shot acquisitions supported with field monitoring. We report online data volume reduction by receiver coil compression and field probe data processing in the order of up to 80% in a setup consisting of a 32-channel array and 16 probes for magnetic field monitoring.Methods
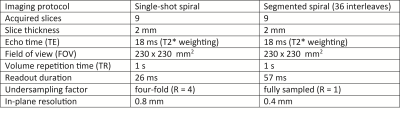
All experiments were performed on a 7T human scanner (Phillips Healthcare, Best, The Netherlands). Data were collected using a concurrent imaging and monitoring setup as in18. It consists of a commercially available quadrature-transmit coil surrounding a 32-channel head receive array (Nova Medical, Wilmington, USA) and in-between an integrated array of 16 19F NMR field probes. The probes were operated using standalone versions of the direct digital synthesis (DDS) and transmit-receive (T/R) box presented in19 and acquired using an in-bore receiver module17. Additional two 16-channel in-bore receivers17 were used to digitize the coil signals. All receivers were phase-frequency locked to the processing platform through the back-channel of the optical link. The platform was used to fuse the coil data streams and compress it down to 12 virtual coils in real-time before storing using a set of embedded matrix-vector multipliers. The probe phases were calculated using the CORDIC20 algorithm and fitted to a second-order spherical-harmonic field model10. After unwrapping and off-resonance correction, phases were transformed into k-space trajectories using a second matrix-vector multiplier. Since the gradient fields have a bandwidth of up to 30kHz, a decimation filter was employed for trajectory down-sampling. All operations have been performed in the field programmable gate array portion of the platform SoC (Xilinx, San Jose, USA) without additional computational resources. A picture of the used hardware without coils and probe front-end is given in Fig. 1. Two scan protocols were employed: segmented spirals18 for anatomical imaging to demonstrate the fidelity of the receive chains and single-shot spirals as used in fMRI21,22. The imaging parameters are given in Table 1. Image reconstruction was performed by inversion of the expanded signal model23,24,18 including static off-resonance and coil sensitivity maps obtained with a separate multi-gradient-echo spin-warp scan, as well as zero and first order k-space trajectories. The coil compression matrix was calculated from the sense maps according to25.Results
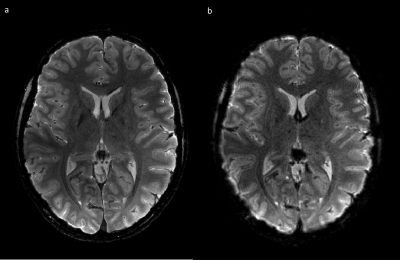
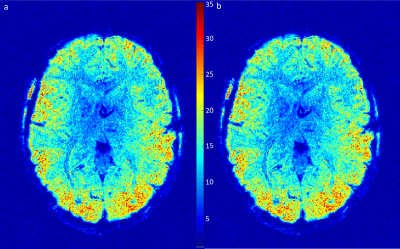
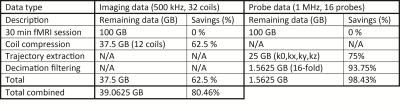
Fig. 2 shows a single slice of the acquired multi- and single-shot spirals. The multi-shot image shows a high consistency between the interleaves and data modalities. The single-shot, as commonly used in fMRI, is of high quality and provides a fine resolution. Fig. 3 shows temporal SNR (tSNR) plots for a time series acquisition of the single-shot spiral. Two images are shown - raw and compressed data. Coil compression results in a high degree of data savings (62.5% in the shown case) with 3% SNR penalty. All images were reconstructed with trajectories calculated in hardware resulting in 98.43% data savings. For a typical 30 min fMRI session 200GB raw data are reduced down to 40GB saving 80.46% in total as shown in Table 2.Discussion
The steady increase in data volume has become a significant problem with data transfer becoming the limiting factor in reconstruction even with cutting-edge hardware. It is demonstrated that data processing already at the level of the acquisition system can drastically reduce the data volume. As demonstrated for the case of high temporal and spatial resolution single-shot data acquisition, this reduction of data volume comes at a negligible loss of image quality.Acknowledgements
No acknowledgement found.References
1. Sodickson DK, et al. Simultaneous acquisition of spatial harmonics (SMASH): Fast imaging with radiofrequency coil arrays. MRM 1997;38(4):591–603.
2. Pruessmann KP, et al. SENSE: Sensitivity encoding for fast MRI. MRM 1999;42(5):952–962
3. Griswold MA, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). MRM 2002;47(6):1202–1210
4. Setsompop K, et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. MRM 2012;67(5):1210–1224
5. Hardy CJ, et al. 128-channel body MRI with a flexible high-density receiver-coil array. JMRI 2008;28(5):1219–1225
6. Schmitt M, et al. A 128-channel receive-only cardiac coil for highly accelerated cardiac MRI at 3 Tesla. MRM 2008;59(6):1431–1439
7. Wiggins GC, et al. 96-Channel receive-only head coil for 3 Tesla: Design optimization and evaluation. MRM 2009;62(3):754–762
8. Ooi MB, et al. Prospective real-time correction for arbitrary head motion using active markers. MRM 2009;62(4):943–954
9. Haeberlin M, et al. Real-time motion correction using gradient tones and head-mounted NMR field probes. MRM 2015;74(3):647–660
10. Barmet C, et al. Spatiotemporal magnetic field monitoring for MR. MRM 2008;60(1):187–197
11. Zaitsev M, et al. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. Neuroimage 2006;31(3):1038–1050
12. Tremblay M, et al. Retrospective coregistration of functional magnetic resonance imaging data using external monitoring. MRM 2005;53(1):141–149
13. Andrews-Shigaki BC, et al. Prospective motion correction for magnetic resonance spectroscopy using single camera retro-grate reflector optical tracking. JMRI 2011;33(2):498–504
14. Felblinger J, et al. Recordings of eye movements for stimulus control during fMRI by means of electro-oculographic methods. MRM 1996;36(3):410–414
15. Marjanovic J, et al. An FPGA Based Real-Time Data Processing Structure – Application to Real-Time Array Coil Data Compression. Proc. ISMRM 2016
16. Marjanovic J, et al. Distributed receivers with hardware-accelerated signal processing: Synchronous acquisition of image data and k-space trajectories. Proc. ISMRM 2017
17. Reber J, et al. In-Bore Broadband Array Receivers with Optical Transmission. Proc ISMRM 2014
18. Kasper L, et al. Rapid anatomical brain imaging using spiral acquisition and an expanded signal model,” Neuroimage 2017:1–13
19. Dietrich BE, et al. A field camera for MR sequence monitoring and system analysis. MRM 2016;75(4):1831–1840
20. Volder JE, The CORDIC Trigonometric Computing Technique. IRE Trans. Electron. Comput. 1959;EC-8(3):330–334
21. Glover GH, et al. Self-navigated spiral fMRI: Interleaved versus single-shot. MRM 1998;39:361–368
22. Noll DC, et al. Spiral K-space MR imaging of cortical activation. JMRI 1995;5:49–56
23. Pruessmann KP, et al. Advances in sensitivity encoding with arbitrary k -space trajectories. MRM 2001;46:638–651
24. Kasper L, et al. Matched-filter acquisition for BOLD fMRI. Neuroimage 2014;100C:145–160
25. Buehrer M, et al. Array compression for MRI with large coil arrays. MRM 2007; 57(6):1131–1139
Figures