5451
fMRI with Low Acoustic Noise using Variable-Blipped EPI with Readout Segmentation1Center of medical physics and engineering, University of Erlangen-Nuremberg, Erlangen, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3University of Glasgow, Glasgow, United Kingdom, 4Siemens Healthcare United Kingdom, Glasgow, United Kingdom, 5University of Erlangen-Nuremberg, Erlangen, Germany, 6Siemens Shenzen Magnetic Resonance Ltd., Shenzen, China
Synopsis
Echo-Planar-Imaging is widely used for fMRI, but generates a high level of acoustic noise This could potentially affect activation power, especially for auditory experiments. One established way to reduce acoustic noise is to use a sinusoidal readout gradient combined with a constant phase-encoding gradient. However, this data-sampling scheme is only suitable for lower acceleration factors. This study investigates an alternative EPI sampling scheme with similar acoustic-noise properties that can be used with high acceleration factors in both phase-encoding and slice-encoding directions. Acoustic noise is further reduced by using readout segmentation to reduce the readout gradient amplitude and slew rate.
Introduction
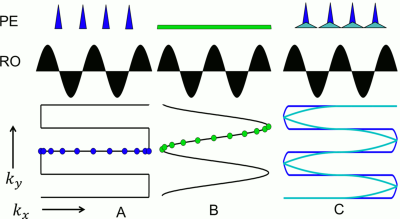
Typically, standard echo-planar imaging (EPI)1 produces high acoustic noise due to the fast gradient switching used during the readout. This is especially the case when using a trapezoidal readout (RO) and blipped phase-encoding (PE) gradient2. The high acoustic noise can be reduced by using a sinusoidal RO and a constant PE gradient3, which can result in a higher level of activation4. However, the resulting non-Cartesian k-space trajectory provides challenges when combined with parallel imaging (PI). As an alternative, this study explored the use of a variable-blipped (VB) EPI acquisition scheme, in which blipped gradients are elongated to reduce their contribution to acoustic noise without causing a major change to the k-space trajectory, even when PI is used with high acceleration factors. The modified EPI sampling scheme was combined with RO segmentation5 to further reduce acoustic noise6.Methods
All data were acquired with a prototype sequence on a MAGNETOM Terra 7T system (Siemens Healthcare GmbH, Erlangen) with a single-channel-transmit, 32-channel-receive head coil (Nova Medical, Wilmington MA, USA). Approval for the study was granted by the local ethics committee.
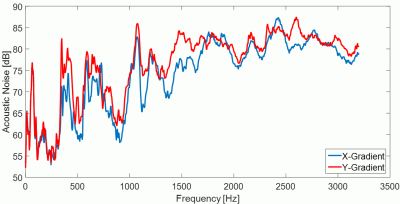
By prolonging the duration of the PE blips in the VB case, the amplitude and slew rate decreases, leading to reduced acoustic noise (compare Fig. 1). In contrast to standard blipped EPI, data are acquired throughout the blips with the VB method. This results in non-Cartesian parts of the trajectory at the k-space edges (compare Fig. 1) which can be reconstructed with non-Cartesian PI methods, like ESPIRiT7. If the non-Cartesian deviations are sufficiently small, Cartesian PI methods, like GRAPPA8, can also be used with acceptable results. The same concepts also apply for slice GRAPPA9 due to the similarity between a 3D k-space and an SMS-accelerated acquisition10. Simulations using a Shepp-Logan phantom as well as measurements were performed to investigate the effect of data sampling during the PE and slice-encoding blips. In addition, acoustic noise measurements were performed to determine the effect of the duration of the blipped-PE gradient gradient on the level of acoustic noise.
The VB sampling scheme was combined with RO segmentation, which provides the option to match the echo spacing to the gradient frequency response function (FRF) of the MR system being used (compare Fig. 2).
A finger-tapping fMRI experiment was performed with a spatial resolution of 1mm. The overall scanning time was the same for both acquisitions with a reduced slice coverage for the RO-segmented scheme. Data analysis was performed using Brain-Voyager (Brain Innovation, Maastricht, Netherlands) with a minimal post-processing protocol consisting of motion correction, high-pass filtering and slice-time correction.
Results
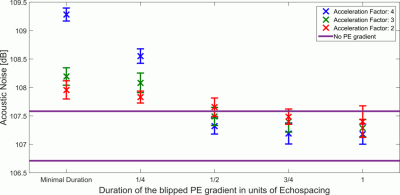
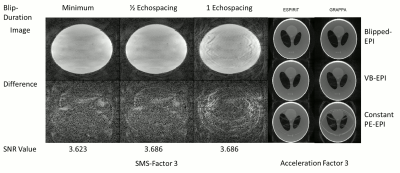
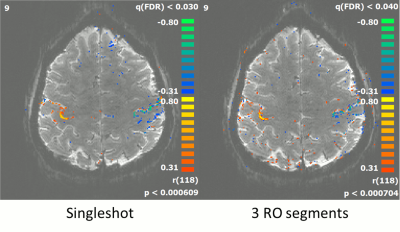
As shown in fig. 3, for blip durations greater than ½ echo-spacing, the acoustic noise contribution from the gradient blips falls within the range measured without PE gradients (denoted by the horizontal purple lines). The simulation results, shown in fig. 4 demonstrate the superior PI performance of VB-EPI compared to constant-PE EPI. Compared to standard blipped EPI, image quality is only slightly degraded, with shorter blip durations giving in general a higher image quality. In the slice-encoding direction, the images with ½ echo-spacing don’t exhibit additional artefacts when compared to images corresponding to a minimum blip duration. Extending the blip duration to one echo spacing however results in a significant increase in the level of artefact. Fig 5 demonstrates that RO segmentation, Cartesian GRAPPA and Slice GRAPPA can be successfully combined with VB-EPI and provides an activation pattern that is very similar to the single-shot case.Discussion
With the VB-EPI method, there is a trade-off between acoustic noise reduction and image quality. From the data acquired in this study, the optimal blip duration was found to be around ½ echo-spacing. The method allows EPI to be performed with reduced acoustic noise, even when it is combined with PI and blipped CAIPIRINHA. The reconstruction quality is in general superior to constant-PE EPI, whereby the same acoustic noise was measured for both sequences. Furthermore, the method can be combined with RO segmentation to provide a further reduction in acoustic noise, albeit at the cost of a longer TR or reduced slice coverage.Conclusion
This study has described a new approach to EPI image acquisition, which provides additional control of acoustic noise in fMRI experiments. Critically, the new technique provides full support for scan acceleration using both in-plane parallel imaging and simultaneous multislice methods. Preliminary fMRI studies of the motor cortex have demonstrated the ability of the technique to provide high quality images and associated activation maps.Acknowledgements
We would like to thank Dr. Annette Stein for helping conduct the acoustic noise measurements and Dr. Mario Zeller for helpful discussions.References
1. Mansfield P. Multi-planar image formation using NMR spin echoes. J. Phys. C: Solid State Phys. 1977;10(3):L55
2. Ravicz ME, Melcher JR, Kiang NYS, Acoustic noise during functional magnetic resonance imaging. The Journal of the Acoustical Society of America, 2000;108(4):1683-1696.
3. Schmitter S, Diesch E, Amann M, Kroll A, Moayer M, Schad LR. Silent echo-planar imaging for auditory FMRI. Magma, 2008;21(5):317-25.
4. Peelle JE, Eason RJ, Schmitter S, Schwarzbauer C, Davis MH, Evaluating an acoustically quiet EPI sequence for use in fMRI studies of speech and auditory processing. Neuroimage, 2010;52(4):1410-9.
5. Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009;62(2):468-75.
6. Ott M, Blaimer M, Grodzki DM, Breuer FA, Roesch J, Dörfler A, Heismann B, Jakob PM. Acoustic-noise-optimized diffusion-weighted imaging. Magma, 2015;28(6):511-21.
7. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT--an eigenvalue approach to autocalibrating parallel MRI: where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001
8. Griswold M, Jakob P, Heidemann R, Nittka M, Jellus V, Wang J, Kiefer B, HaaseA. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn Reson Med. 2002;47:1202-10.
9. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-24.
10. Zahneisen B, Poser BA, Ernst T, Stenger VA. Three-dimensional Fourier encoding of simultaneously excited slices: generalized acquisition and reconstruction framework. Magn Reson Med. 2014;71(6):2071-81.
Figures