5447
StImulus Locked K-space shuffling (SILK) for ultra-high resolution fMRI1Radiology, San Francisco VA Health Care System, San Francisco, CA, United States, 2Center for Imaging of Neurodegenerative Diseases, University of California at San Francisco, San Francisco, CA, United States, 3Helen Wills Neuroscience Institute, University of California at Berkeley, Berkeley, CA, United States, 4Advanced MRI Technologies, Sebastopol, CA, United States
Synopsis
Traditional EPI fMRI methods for acquiring sub-mm ultra-high resolution images require longer echo trains, resulting in SNR losses due to longer TEs, as well as, increased blurring in the phase encode (PE) direction, geometric distortion, and susceptibility dropout. StImulus Locked K-space shuffling or SILK-fMRI (e.g. in the context of 3D FLASH imaging) does not suffer from the above limitations but has never before been applied to fMRI. Here we demonstrate the feasibility of SILK fMRI vs. traditional EPI for 0.35 mm ultra-high resolution fMRI at 7T.
Purpose
Traditional EPI fMRI methods for acquiring sub-mm ultra-high resolution images require longer echo trains, resulting in SNR losses due to longer TEs, as well as, increased blurring in the phase encode (PE) direction, geometric distortion, and susceptibility dropout. While these challenges can be mitigated through use of in-plane acceleration techniques (e.g. GRAPPA or Partial Fourier), such techniques are not without drawbacks1. Barring the use of custom gradient coils or high-density receiver array coils2, it is generally thought that voxel sizes smaller than 0.5-0.8 mm are not feasible, precluding study of mesoscale functional organization. In the context of 3D FLASH imaging, StImulus Locked K-space shuffling or SILK-fMRI (Provisional Patent #62526296) does not suffer from the above limitations but has typically not been considered for fMRI, in part, due to the limiting traditional view that the volume imaging time (i.e. TR) for fMRI must be ~2 s or at least no longer than a given brain state (although there are some special fMRI applications for traditional 3D FLASH where imaging of V1 with a volume TR of 91 s is acceptable3). Here we demonstrate the feasibility of SILK fMRI to achieve an effective temporal resolution of ~2 s while acquiring 0.35 mm ultra-high resolution fMRI at 7T.Methods
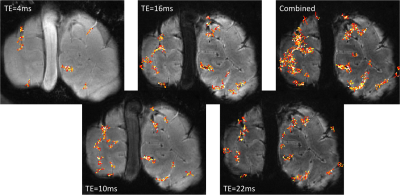
Ultra-high resolution fMRI was acquired using 3D GRE FLASH SILK fMRI (TA=7 min, TR=22.4 ms, FA=8 deg, TE=16 ms, # of measurements = 16, 0.35x0.35x0.7 mm3 voxels, FOV=89x89x11.2 mm3, IPAT=3, PE=HF, slice PF=6/8, phase PE=6/8, BW=149 Hz/px) as well as SLIDER fMRI4 (TA=6 min, TR=6000 ms, FA=90 deg, TE=38 ms, 0.35 mm nominally isotropic voxels, SLIDER=2, FOV = 78x89x12.6 mm3, IPAT=2, PE=HF, PF=5/8, BW=550 Hz/px, ES=1.91 ms). A pilot Multi-Echo SILK fMRI dataset was also acquired (same parameters as SILK fMRI above except: TR=27 ms, FA=10 deg, TEs =4, 10, 16, 22, BW=300 Hz/px, # of measurements = 9). Custom modification of the SLIDER fMRI sequence was required to enable BW<750 Hz/px (e.g. voxels < 0.45 mm in-plane). A custom 8 ch RX 1ch TX surface coil5 was used for additional SNR on a Siemens MAGNETOM 7T body gradient system (70 mT/m, 200 mT/m/s). One subject was scanned while being presented hemi-field specific 8 Hz flickering checkerboard visual stimuli of period 36 s (i.e. 18 s left hemi-field stimulation followed by 18 s right hemi-field stimulation). With 16 measurements, the effective temporal resolution or TR of the SILK fMRI dataset was 36 s / 16 = 2.25 s.Results
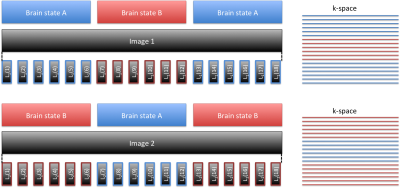
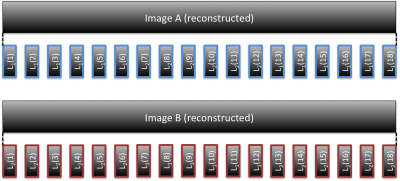
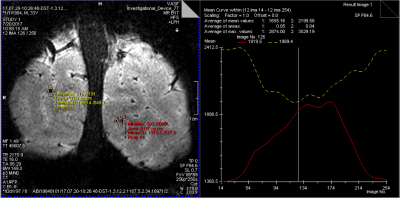
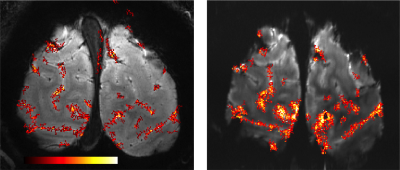
Figure 1 depicts the acquisitions strategy for the proposed Stimulus locked k-space shuffling method. In this example, the volume imaging time is three times as long as the brain state (i.e. block) duration. By acquiring multiple images while varying the timing of the brain states, complete sets of k-space lines can be acquired for each brain state. Figure 2 shows how reshuffling k-space lines across multiple image acquisitions, according to brain state, can provide reconstructed images of individual brain states. Figure 3 shows example BOLD time-courses for two separate ROIs for a single 7 min acquisition of SILK fMRI. Figure 4 shows the stimulus CNR for SILK fMRI (left) and EPI (right) overlaid onto the across-run temporal mean image. Figure 5 shows stimulus CNR for a Multi-Echo SILK fMRI dataset.Conclusions
SILK fMRI is able to detect BOLD activations with minimal geometric distortions, blurring, and susceptibility dropout, which will be critical for studying mesoscale functional organization in conjunction with T1w and T2w anatomical images. Although the nominal in-plane resolution and amount of head motion was the same in both SILK & EPI datasets, the SILK data shows impressive retention of resolution - enabling visualization of small diving veins not apparent in the EPI image. In this preliminary study, SILK CNR is weaker than EPI CNR, in part due to the shorter TE in the SILK dataset (16 vs. 38 ms) and relatively short TR, which limits T1 relaxation. Additional optimization of the Multi-Echo SILK protocol may further improve SILK CNR without resorting to extremely low readout bandwidths. SILK fMRI is expected to be particularly useful in regions where EPI suffers from strong susceptibility dropout and distortion (e.g. orbital frontal and ventral temporal cortex). Importantly SILK as well as Multi-Echo SILK fMRI data can also be processed using SWI or QSM techniques for enhanced BOLD CNR and identification/exclusion of large and mid-sized veins6.Acknowledgements
NIH BRAIN Initiative grants 1R24MH106096, 5R01MH111444, and 1U01EB025162-01.References
1) Vu et al. Neuroimage (2017): Tradeoffs in pushing the spatial resolution of fMRI for the 7T Human Connectome Project.
2) Feinberg, Vu, & Beckett. Neuroimage (2017): Pushing the limits of ultra-high resolution human brain imaging with SMS-EPI demonstrated for columnar level fMRI.
3) Koopmans et al. Neuroimage (2011): Multi-echo fMRI of the cortical laminae in humans at 7 T.
4) Vu et al. Neuroimage (2017): Evaluation of Slice Dithered Enhanced Resolution Simultaneous MultiSlice (SLIDER-SMS) for human fMRI.
5) Beckett et al. ISMRM (2016): Assessment of coil arrays with small loop diameter at 7T for micron-scale resolution fMRI of human neocortex.
6) Vu and Gallant. Frontiers Neuroscience (2015): Using a novel source-localized phase regressor technique for evaluation of the vascular contribution to semantic category area localization in BOLD fMRI.