5443
Distortion-matched high-resolution reduced-FoV functional and diffusion MRI of the human brainstem at 7T1Department of Physics, University of Milan, Milano, Italy, 2CUBRIC, School of Psychology, Cardiff University, Cardiff, United Kingdom, 3Siemens Healthcare Ltd, Frimley, Camberley, United Kingdom, 4Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy, 5Brain Connectivity Center, C. Mondino National Neurological Institute, Pavia, Italy, 6Department of Physics, University of Pavia, Pavia, Italy, 7Department of Electrical, Computer and Biomedical Engineering, University of Pavia, Pavia, Italy, 8Queen Square MS Centre, UCL Institute of Neurology, Faculty of Brain Sciences, University College London, London, United Kingdom, 9Brain MRI 3T Research Centre, C. Mondino National Neurological Institute, Pavia, Italy
Synopsis
The brainstem plays a key role in the central nervous system, but its proximity to the oral cavity and physiological noise sources constitute major limitations for EPI imaging. We compared image quality from full and reduced-FoV spin-echo EPI sequences at ultrahigh field. Compared to standard EPI acquisitions, reduced-FoV techniques confer considerable benefits for brainstem imaging in terms of shortening TE, increasing signal-to-noise ratio and mitigating distortions. We exploited these advantages for 7T distortion-matched functional and diffusion imaging of the brainstem using ZOOM-EPI. A finger tapping task resulted in significant activations in regions corresponding to the cuneate nucleus and the pyramidal decussation.
Introduction
The brainstem plays a central role in the central nervous system and comprises a large number of small relay nuclei and intricate fibre geometry1. Despite its functional and physiological importance, MRI investigation of the brainstem has always been hampered by many factors. Along with the small size of its constituents and its susceptibility to physiological noise2, the proximity of the oral cavity constitutes a major limitation for brainstem MRI.
Echo planar imaging (EPI) is the most commonly-used sequence for functional and diffusion studies, but it is strongly affected by magnetic susceptibility gradients at air-tissue interfaces, which give rise to severe image distortions in the phase encoding direction and thus limit EPI applicability. Ultrahigh field MRI can provide higher signal, resolution and contrast, but it also leads to even larger susceptibility-induced distortion. To compensate for this, the use of reduced-FoV techniques3 can shorten the EPI echo train length, hence changing the pixel bandwidth, and reducing the distortions.
In this work we compared image quality from full and reduced-FoV spin-echo EPI of the human brainstem at ultrahigh field. The optimal protocol in our judgement was used for distortion-matching functional and diffusion imaging of the brainstem.
Methods
Image acquisition: One right-handed healthy subject (male, age 47) participated in this study. Measurements were performed on a whole-body 7T research MR-system (Siemens Healthcare GmbH, Erlangen, Germany), with a 32-channel head receive/volume transmit coil.
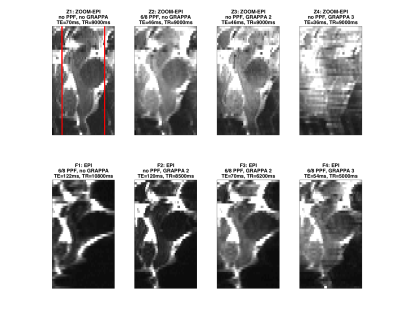
Distortion evaluation: As shown in Figure 2, eight different combinations of full (four protocols labeled F1-F4) and reduced-FoV (four protocols labeled Z1-Z4) imaging, phase partial Fourier (PPF) and parallel imaging were tested with full coverage of the brainstem. For full/reduced-FoV imaging a single shot spin-echo EPI/ZOOM-EPI4 sequence was used: imaging FoV 220x220mm2/52.5x70.0mm2, acquisition matrix 200x200/48x64, ZOOM FoV 35.0x70.0mm2, 2 slices ZOOM gap, AP PE direction, 1.1x1.1x2.0 mm3 resolution, 46 slices, BW=1860Hz/Px (1924Hz/Px), 8 averages. Slice centre and orientation were maintained from reduced to full-FoV acquisition.
fMRI and DWI: The subject performed a finger tapping task (right hand, blocks duration: 30s). The Z2 protocol was adopted for fMRI acquisition (150 volumes) and a b=50s/mm2 monopolar diffusion weighting was applied along the z-axis to reduce signal originating from larger vessels. Heart beat and respiration were continuously recorded throughout the acquisition. Identical geometric parameters were maintained for matched monopolar diffusion weighted imaging (DWI): TE=60ms, b=500 and 1000s/mm2, respectively 30 and 60 isotropically distributed directions5. The protocol included also two b=0s/mm2 volumes with reversed PE direction for susceptibility distortion correction and a whole brain T1w MP2RAGE for structural reference (0.7 isotropic resolution, TR=4.5s, TE=2.26ms). The scan session lasted approximately 1h.
Data analysis: fMRI data pre-processing included slice-by-slice RETROICOR6 correction for physiological noise. Slice timing, realignment, smoothing and data processing were performed using SPM (http://www.fil.ion.ucl.ac.uk/spm/). DWI data were corrected for susceptibility-induced distortion, motion and eddy currents using FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Constrained spherical deconvolution and probabilistic tractography7 followed diffusion tensor estimation.
Results and discussion
Figures 2 and 3 show the influence of acquisition parameters in terms of distortions. Full FoV images were cropped to match ZOOM acquisition FoV for display purposes.
High resolution imaging of the brainstem can be crucial to correctly identify small structures and can help to disentangle the BOLD signal of the nuclei from surrounding physiological noise sources. On the other hand, larger acquisition matrices needed to achieve high resolution necessitate longer TEs with consequent signal loss and increased distortions. Reduced-FoV imaging techniques like ZOOM-EPI allow us to eliminate unnecessary brain regions from the acquisition and thus reduce the matrix size.
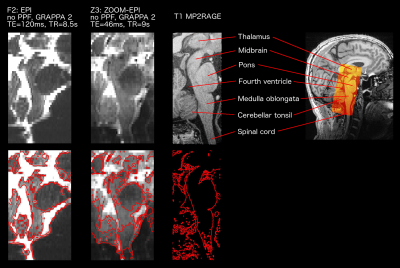
Considering results in terms of signal-to-noise ratio, reduced distortions and absence of reconstruction artefacts from parallel imaging, the Z2 protocol was judged to be the best compromise for fMRI and DWI.
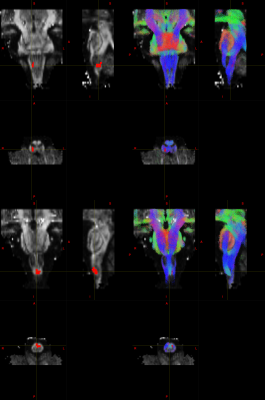
The lack of contrast between grey and white matter in the brainstem in T2(*)w and T1w images constitutes a further obstacle for nuclei identification: for this reason, FA maps have been used to locate nuclei8 and fibre tracking provide additional anatomical information (Fig.5). Furthermore, advanced microstructural models may provide new metrics that provide enhanced characterization of brainstem nuclei.
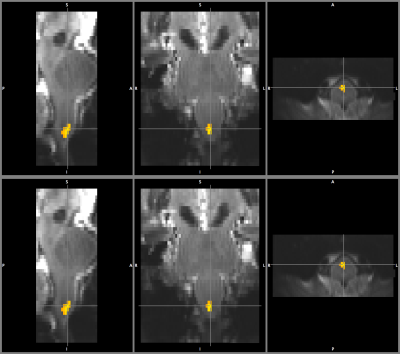
In line with reported findings9,10, our fMRI analysis shows a significant activation in the medulla oblongata in correspondence to the pyramidal decussation (motor pathway) and the ipsilateral (right) cuneate nucleus (Fig.4), which is pertaining to the sensory pathway involved in fine touch and proprioception.
Conclusions
Compared to standard EPI acquisitions, reduced-FoV techniques confer considerable benefits for brainstem imaging in terms of shortening TE, increasing signal-to-noise ratio and mitigating distortions. These advantages can be successfully exploited for high resolution functional and diffusion imaging of the brainstem at ultrahigh fields.Acknowledgements
We would like to thank the Medical Research Council, UK for the grant (MR/M008932/1) that supported this work.References
1. Sclocco, Roberta, et al. "Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI." NeuroImage (2017).
2. Brooks, Jonathan CW, et al. "Physiological noise in brainstem FMRI." Frontiers in human neuroscience 7 (2013).
3. Wargo, Christopher J., Jay Moore, and John C. Gore. "A comparison and evaluation of reduced-FOV methods for multi-slice 7T human imaging." Magnetic resonance imaging 31.8 (2013): 1349-1359.
4. Wheeler‐Kingshott, Claudia AM, et al. "ADC mapping of the human optic nerve: Increased resolution, coverage, and reliability with CSF‐suppressed ZOOM‐EPI." Magnetic resonance in medicine 47.1 (2002): 24-31.
5. Caruyer, Emmanuel, et al. "Design of multishell sampling schemes with uniform coverage in diffusion MRI." Magnetic resonance in medicine 69.6 (2013): 1534-1540.
6. Glover, Gary H., Tie‐Qiang Li, and David Ress. "Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR." Magnetic resonance in medicine 44.1 (2000): 162-167.
7. Tournier, J., Fernando Calamante, and Alan Connelly. "MRtrix: diffusion tractography in crossing fiber regions." International Journal of Imaging Systems and Technology 22.1 (2012): 53-66.
8. Bianciardi, Marta, et al. "Toward an in vivo neuroimaging template of human brainstem nuclei of the ascending arousal, autonomic, and motor systems." Brain connectivity 5.10 (2015): 597-607.
9. Faull, Olivia K., et al. "Functional subdivision of the human periaqueductal grey in respiratory control using 7tesla fMRI." Neuroimage 113 (2015): 356-364.
10. Pattinson, Kyle TS, et al. "Opioids depress cortical centers responsible for the volitional control of respiration." Journal of Neuroscience 29.25 (2009): 8177-8186.
Figures