5440
Gain of temporal signal-to-noise ratio with pTx universal pulses and the whole-brain fMRI 3D-GE EPI sequence at 7T1NeuroSpin, CEA, Saclay, France, 2DZNE, Bonn, Germany
Synopsis
The volumetric 3D-EPI sequence has already shown great promise for fMRI studies due to potentially increased temporal SNR (tSNR) and lower energy demands compared to 2D multi-slice acquisition schemes. For whole-brain studies the tSNR yet can suffer from sub-optimal flip angles due to RF inhomogeneity. Parallel transmission (pTx) has been shown to be a very powerful technology to mitigate these effects but has been barely used in routine due to a cumbersome calibration procedure. Here, we use Universal Pulses to skip entirely the latter step and increase locally the tSNR at 7T in the 3D-EPI GE sequence.
Introduction
The volumetric 3D-EPI sequence is particularly attractive for whole-brain fMRI studies due to increased SNR compared to multi-slice 2D acquisition schemes1. Temporal SNR (tSNR) as an indicator of sensitivity for detection of neuronal activation yet can suffer locally from sub-optimal flip angle excitations2. Here we demonstrate the application of pTx universal pulses3,4,5 to restore the tSNR in B1+-deprived regions at 7T, without the usual and cumbersome parallel transmission (pTx) calibration.Methods
Measurements were performed at 7T on a Magnetom scanner (Siemens Healthcare, Erlangen, Germany) and with the 8Tx-8Rx Rapid- Biomed head coil (Rapid biomedical, Rimpar, Germany). The sequence used was a custom 3D-EPI6 sequence with sagittal slice orientation, TE=21 ms, 1.5×1.5×1.5 mm3 resolution, matrix 112×140×140, iPAT acceleration factor of 2×2 along the phase and partition encoding directions respectively, PF=6/8 along the PE direction, leading to a volume TR of 2.4s with whole-brain coverage. The universal, non-selective, pulse was designed on a previously acquired database of 10 B1 and ΔB0 maps4 with a kT-point pulse parametrization7 and with target flip angle (FA) of 12° corresponding to the Ernst angle for gray matter. The pulse design algorithm consisted of minimizing the FA Normalized Root Mean Square Error (NRMSE) averaged over the database subjects, under explicit hardware and SAR constraints, and with simultaneous optimization of the k-space trajectory8. The total duration of the kT-points UP was varied in a prior simulation study over the database subjects to achieve water selective excitation and potentially provide fat artefact-free images9. Two series of 100 volumes were acquired with the 3D-EPI sequence on four different healthy volunteers by blindly applying the pre-computed kT-points UP and a single CP-mode 2ms square pulse, respectively. tSNR was computed for each voxel in the brain by calculating the mean magnitude signal of the time-series divided by its standard deviation, after correcting for a linear drift and after volume realignment using FSL10. Finally, B1 and ΔB0 maps were acquired for each subject for retrospective control of the universal pulse performance with Bloch simulations.Results
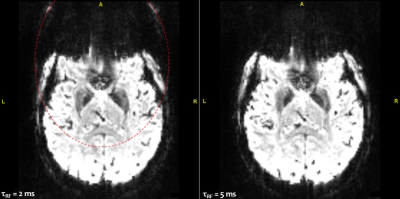
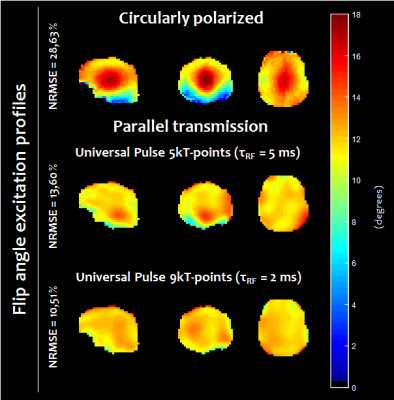
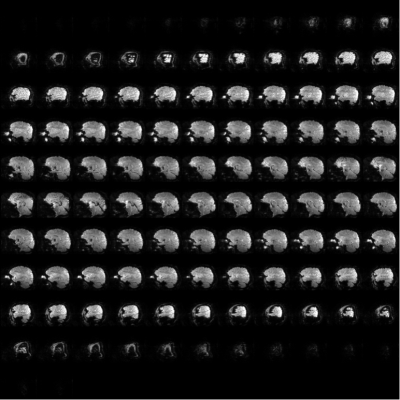
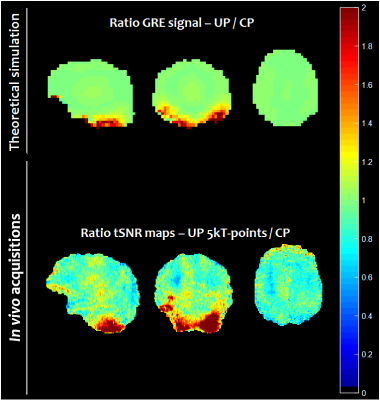
Figure 1 illustrates the gain of increasing the pulse duration from 2 ms (9 kT-points ~ 0.2 ms/sub-pulse) to 5 ms (5 kT-points ~ 1.0 ms/sub-pulse) to remove the chemical shift artefact. This was confirmed by our simulations which indicated that for a 5 ms pulse duration, the mean FA over the database subjects for fat was 8 times smaller than for water, while they were comparable for a 2 ms pulse. Figure 2 reports the computed FA maps for the CP and UP excitations, based on the measured subject-specific field maps. Retrospectively, it could be thereby determined that the UP achieved 13.6% NRMSE compared to 28.6% in CP mode. Due to B0 inhomogeneity however, the 5 ms pulse duration for water selective excitation came at the cost of slightly poorer FA uniformity compared to a 2 ms kT-point pulse (13.6% versus 10.5%). The shorter solution on the other hand was not retained for the experiments because of the more pronounced chemical-shift artefact. Figure 3 shows for one subject two series of 25 sagittal slices obtained with the UP and CP modes respectively, clearly illustrating the signal gain obtained in the cerebellum and the temporal lobes with the UP. Figure 4 shows for the same volunteer the resulting measured versus predicted tSNR gain (in the thermal noise dominated regime), the latter being computed by taking the FA maps and GRE signal equation with uniform T1. A tSNR improvement of 20 % on average over the whole brain and up to a factor of 4 locally when using UPs could be observed experimentally. Similar results were obtained for the three other volunteers.Discussion and conclusion
A gain by up to a factor of 4 in tSNR in the cerebellum and temporal lobes with the 3D-EPI sequence was obtained through the use of pre-computed pTx universal pulses. The pulses were designed prior to the acquisitions to be robust against intersubject field map variability and thus could be blindly applied potentially on any subject, i.e. without any subject-specific pTx calibration. To minimize chemical-shift artefacts, a recently proposed water excitation technique9 based on a single square pulse was successfully integrated into the pTx UP. Apart from the cerebellum and temporal lobes, the tSNR gain compared to the CP mode was found to be insignificant and can be explained by the flattening of the signal around the Ernst angle for small interpulse repetition times. More widespread gains of tSNR are expected for acquisitions with higher FA and larger repetition times such as multi-slice GRE-EPI sequences.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Program, Proof of Concept Grant Agreement n. 700812.References
[1] B.A. Poser, P.J. Koopmans, T. Witzel, L.L. Wald, M. Barth. Three dimensional echo-planar imaging at 7 Tesla. NeuroImage 51:261-266 (2010).
[2] X. Wu, E. Auerbach, A. Vu, S. Moeller, J. Keith, S. Schmitter, P-F. Van de Moortele, E. Yacoub, K. Ugurbil. High Resolution Resting-State Functional MRI at 7 Tesla Using RF Parallel Transmission. Proc of the 25th Annual meeting of the ISMRM, Honolulu, Hawaii, USA, p 5231 (2017).
[3] V. Gras, A. Vignaud, A. Amadon, D. Le Bihan and N. Boulant. Universal pulses: A new concept for calibration-free parallel transmission. Magn Reson in Med 77:635-643 (2017).
[4] V. Gras, M. Boland, A. Vignaud, G. Ferrand, A. Amadon, F. Mauconduit, D. Le Bihan, T. Stoecker, N. Boulant. Homogeneous non-selective and slice-selective parallel-transmit excitations at 7 Tesla with universal pulses: a validation study on two commercial RF coils. PLoS ONE 12(8): e0183562.
[5] V. Gras, F. Mauconduit, A. Vignaud, A. Amadon, D. Le Bihan, T. Stoecker, N. Boulant. Design of universal kT-point pulses and application to three-dimensional T2-weighted imaging at 7T. doi: 10.1002/mrm.27001.
[6] R. Stirnberg and T. Stöcker. Conventional 2D-EPI or Segmented 3D-EPI? a Temporal SNR Study at 3 and 7 Tesla. Proc of the 22nd Annual meeting of the ISMRM, Milan, Italy, p 868 (2014).
[7] M. A. Cloos, N. Boulant, M. Luong, G. Ferrand, E. Giacomini, D. Le Bihan and A. Amadon. kT-points: Short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn Reson in Med 67:72-80 (2012).
[8] V. Gras, M. Luong, A. Amadon, N. Boulant. Joint design of kT-points trajectories and RF pulses under explicit SAR and power constraints in the large flip angle regime. J of Magn Reson 261:181-189 (2015).
[9] R. Stirnberg, D. Brenner, T. Stöcker, N. J. Shah. Rapid fat suppression for three-dimensional echo planar imaging with minimized specific absorption rate. Magn Reson in Med 76:1517-1523 (2016).
[10] M. Jenkinson, P. Bannister, M. Brady, S. Smith. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage 17:825-841 (2002).
Figures