5439
Spiral fMRI using the Gradient Impulse Response Function for Trajectory Prediction1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, Univeristy of Oxford, Oxford, United Kingdom, 2Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zurich, Switzerland, 3Skope Magnetic Resonance Technologies AG, Zurich, Switzerland
Synopsis
Image artifacts resulting from gradient imperfections remain a challenge in spiral functional MRI. In this work we reconstructed spiral fMRI data using trajectories predicted by the gradient impulse response function (GIRF). The GIRF-reconstruction generates image quality and fMRI results similar to using a fully monitored trajectory. The presented approach requires only a one-time calibration per system, thus the fMRI acquisition is not prolonged or complicated by the acquisition of additional data for correction purposes.
Introduction
Despite its potential for functional imaging1, spiral imaging has not yet become a mainstream fMRI acquisition strategy. The reasons for this include that artifacts caused by gradient imperfections and B0 inhomogeneity are more difficult to correct compared to EPI. It has been shown that concurrent monitoring of the encoding fields using NMR field probes2 enables high quality spiral imaging3,4, but the required setup might not always be available. Therefore we looked at spiral fMRI using trajectories predicted by the gradient impulse response function (GIRF), which can be determined in a one-time calibration step5,6. The image quality and functional results of the reconstructions using GIRF-prediction were compared to reconstructions using a simple delay correction (nominal) and concurrent field monitoring.Methods
All data were acquired on a 7T Philips Achieva system on which the GIRF was measured5 by playing out a set of gradient blips and measuring the field response using a dynamic field camera7. Five minutes of 2D single-shot spiral fMRI data (FOV=190x230, 1mm isotropic resolution, under-sampling factor R=4, 36 slices (1mm+0.2mm gap, FOVz=43mm), TR=2.88s) were acquired on a healthy volunteer. The visual fMRI paradigm consisted of 5s blocks of upper-left/lower-right (ULLR) quarter field stimulation interleaved with upper-right/lower left (URLL) stimulation, separated by 15s of rest. A multi-echo GRE scan (ΔTE=1ms) was collected to estimate coil sensitivities and B0 maps. Concurrent field monitoring2 was performed during all scans using NMR field probes clip-mounted on the 32-channel head receive coil.
Image reconstruction6 (CG-SENSE with multi-frequency interpolation for off-resonance correction) was performed using (a) the delay-corrected nominal k-space trajectory (b) the GIRF-predicted trajectory and (c) the trajectory measured with concurrent field monitoring. For (b) and (c) the imaging data was demodulated by the predicted or measured 0th-order field integrals k0(t).
After image realignment, GLM-analysis was performed using FEAT in FSL8 using no spatial smoothing or clustering. Activation was assessed using z-statistics contrasting ULLR versus URLL ([1 -1] = blue/light blue, [-1 1] = red/yellow).
Results
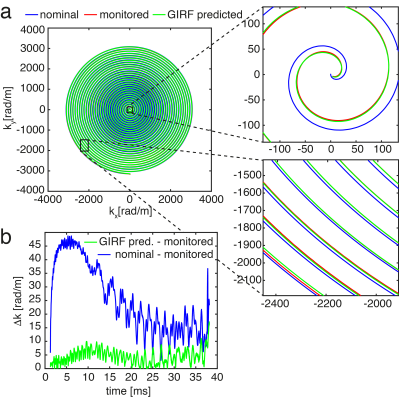
The GIRF-predicted spiral trajectories closely follow the measured ones, while the nominal trajectory deviates substantially, especially at low spatial frequencies (see Fig.1). The mean trajectory error (defined here as the mean absolute error to the monitored trajectory, averaged for all slices and volumes) for the GIRF-predicted trajectory was 74% lower than for the nominal one.
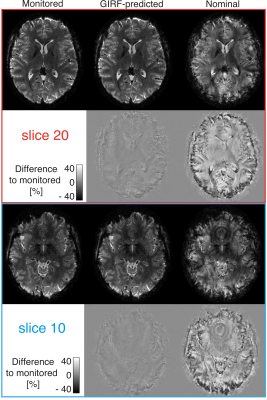
The nominal spiral image is corrupted by blurring and geometric distortion (Fig 2). The GIRF-predicted reconstruction has substantially improved image quality. Small amounts of residual artifacts (a small amount of blurring, shading and ringing) can be observed in the difference images to the monitored reconstruction. The global image artifact level (defined here as the mean absolute error to the monitored reconstruction, averaged over all slices and volumes) was 73% lower for the GIRF-predicted reconstructions compared to the nominal one.
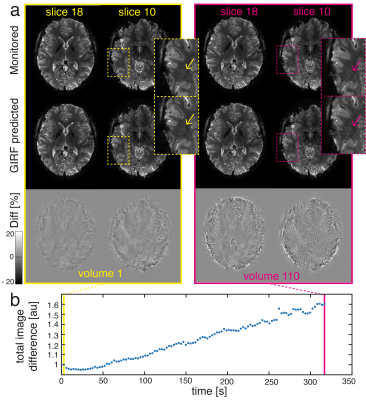
A comparison of the first and final volume in the fMRI time series revealed an increase in image artifacts over time for the GIRF reconstruction (Fig. 3). However, the image artifact reduction compared to nominal still corresponds to 65% in the final volume (compared to 80% for the first volume).
For
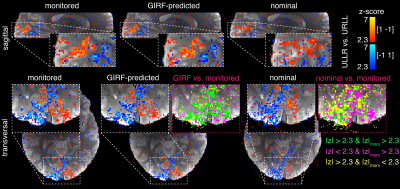
the monitored and GIRF-predicted reconstructions, the spiral fMRI results show good
correspondence of the activation with gray matter architecture (Fig.4), while
the nominal data contain misplaced activation (apparent for example where activation
is crossing white matter boundaries). The activation maps for the GIRF-predicted
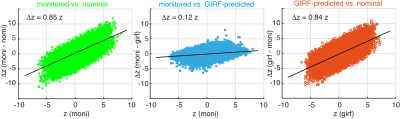
and monitored reconstructions nearly perfectly overlap (Fig. 4), which is confirmed by scatter
plots of z-statistic values for all voxels in a visual cortex grey matter mask (Fig.
5). The GIRF reconstruction provides a large increase in functional contrast (average
increase of 84%), compared to the nominal reconstruction. The monitored
reconstruction in turn provides a small additional improvement over the GIRF
reconstructed z-stats (average increase of 12%).
Discussion
The GIRF-reconstruction generates image quality and fMRI results similar to using a fully monitored trajectory and provides substantial improvement compared to a reconstruction using the delay-corrected nominal trajectory. The residual differences between reconstructions using GIRF-prediction and monitoring are likely due to non-linear or time-dependent effects, which cannot be captured in the GIRF. The increase in artifacts in the GIRF-predicted reconstruction towards the end of the 5-minute acquisition is presumably due to heating of the gradients. This could potentially be tackled with the use of several temperature specific GIRFs9,10.
Conclusion
Using GIRF-predicted trajectories has the potential to enable high-quality spiral fMRI in situations where concurrent monitoring is not available. The presented approach requires only a one-time calibration per system, thus the fMRI acquisition is not prolonged or complicated by the acquisition of additional data for trajectory correction.Acknowledgements
We would like to acknowledge our funding through the Oxford-Brain@McGill-ZNZ Partnership in the Neurosciences. This project has also received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 659263 as well as from NCCR “Neural Plasticity and Repair” at ETH Zurich and the University of Zurich. Technical support from Philips Healthcare is gratefully acknowledged.References
- Glover GH. Spiral imaging in fMRI. NeuroImage. 2012;62(2):706-12
- Barmet C, De Zanche N, Pruessmann KP. Spatiotemporal magnetic field monitoring for MR. Magn Reson Med. 2008;60(1):187-197.
- Wilm BJ, Barmet C, Gross S, et al. Single-shot spiral imaging enabled by an expanded encoding model: Demonstration in diffusion MRI. Magn Reson Med. 2016;77(1):83-91
- Kasper L, Barmet C, Engel M, et al. Single-shot Spiral fMRI at 7 T with High Resolution and Geometric Fidelity. Proceedings of the 23rd Annual Meeting of the Organization for Human Brain Mapping (OHBM) Annual Meeting, 2017, Vancouver
- Vannesjo SJ, Haeberlin M, Kasper L, et al. Gradient system characterization by impulse response measurements with a dynamic field camera. 2013;69(2):583–593
- Vannesjo SJ, Graedel NN, Kasper L, et al. Image reconstruction using a gradient impulse response model for trajectory prediction. Magn Reson Med. 2016;76(1):45-58
- Dietrich BE, Brunner DO, Wilm BJ, et al. A field camera for MR sequence monitoring and system analysis. Magn Reson Med. 2016;75(4):1831-1840.
- Jenkinson M, Beckmann CF, Behrens TEJ, et al. FSL. 2012;62(2):782-790
- Dietrich BE, Reber J, Brunner DO, et al. Analysis and Prediction of Gradient Response Functions under Thermal Load. Proceedings of the ISMRM Annual Meeting 2016, Singapore, no. 3551.
- Dietrich BE, Nussbaum J, Wilm B, et al., Thermal Variation and Temperature-Based Prediction of Gradient Response. Proceedings of the ISMRM Annual Meeting 2017, Hawaii, no. 0079.
Figures