5437
Changes in Resting-State Functional Brain Activity are Correlated with Waning Cognitive Functions in Pediatric HIV1Division of Translational Medicine, Sidra Medicine, Doha, Qatar, 2Department of Radiology and Imaging, Fortis Memorial Research Institute, Gurgaon, India, 3Department of Neurology, King George Medical University, Lucknow, India, 4Department of Microbiology, King George Medical University, Lucknow, India, 5Department of Psychiatry, Sidra Medicine, Doha, Qatar, 6Department of Diagnostic Imaging, Sidra Medicine, Doha, Qatar
Synopsis
We evaluated the functional brain changes in HIV-infected children by mapping the amplitude-of-low-frequency-fluctuations (ALFF) and functional connectivity (FC) using rs-fMRI. Association of these changes with cognitive measures was also explored. ALFF and FC were significantly altered in multiple brain regions involved in auditory, visual, language, motor and sensory activity. The waning cognitive functions in HIV-infected children were associated with the changes in ALFF and FC. These two imaging parameters in association with the cognitive evaluation may provide better understanding of the functional brain activity in HIV-infected children.
Introduction:
Delayed brain development in HIV-infected children may affect the functional brain activity and subsequently cognitive functioning. Recent studies using magnetic-resonance-imaging (MRI) have shown lower gray matter and white matter volumes, changes in cortical thickness, and altered structural connectivity and metabolite levels in the brain of HIV-infected children1-4. To the best of our knowledge, there is no published study evaluating the functional brain changes in HIV-infected children using resting-stage fMRI (rs-fMRI). The current study evaluated the functional brain activity in HIV-infected children by mapping the amplitude of low frequency fluctuations (ALFF) and functional connectivity (FC) using rs-fMRI. Association of ALFF and FC with cognitive measures was also explored.Materials and Methods:
Institutional regulatory board approved the study protocol. 49 HIV-infected children and 23 age/sex matched control were enrolled. Diagnosis of HIV was performed according to the national HIV testing protocol. With informed consent, each child underwent neuropsychological test (NPT) using Revisie Amsterdamse Kinder Intelligentie Test battery, and brain MRI on a 3-T scanner (Signa Hdxt; GE Healthcare, Milwaukee, Wisconsin) using a vendor supplied head coil. Conventional (T1-, T2- and FLAIR), high-resolution T1-weighted and rs-fMRI images were acquired from each subject. Imaging parameters for the rs-fMRI were- TR=2500ms, TE=30ms, FA=90°, number-of-slices=46, slice thickness=3mm, and 120 volumes. 16 HIV-infected children showed hyperintensity on T2-weighted and FLAIR images, and 7 HIV-infected children and 3 control children did not meet the motion criteria (2.5mm translational or 2.5°rotational) and were excluded from the analysis. Data from 26 HIV-infected children and 20 control children were finally included for the statistical analysis (Table 1). The rs-fMRI data was processed by RESTplus software (http://restfmri.net/) in following steps: removal of initial 10 volumes, slice timing, motion correction, coregistered and normalized to MNI space using dartel, and normalized parameters were applied on rs-fMRI images followed by resampling (3mm) and smoothing (6mm) with FWHM isotropic Gaussian kernel. Linear trend was removed and images were band-pass filtered (0.01-0.08Hz). Linear regression with six parameters obtained by motion correction, mean signals from CSF, averaged white matter, and averaged signals across whole brain were used for removing the cofounding effect, and then ALFF maps were constructed. Brain areas showing significant difference on ALFF between two groups were selected as seed regions. The FC maps between whole brain and the selected seed regions were generated by voxel-wise partial correlation coefficients.Statistical Analysis:
Demographic variables between two groups were compared either by Chi-square test or independent student t-test. The NPT scores were assessed using an independent student t-test. Two-sample t-test was performed for the ALFF and FC between two groups. AlphaSim method5 which employs Monte Carlo simulation was used for multiple comparisons correction both for ALFF and FC by applying following thresholds- 10,000 iterations, p<0.005 both at voxel and cluster level (minimum 35 voxels). The ALFF and FC maps were further corrected using age and gender as covariates (Table 2). Pearson's correlation was performed between rs-fMRI parameters and NPT scores.Results:
Demographic, neuropsychological and clinical information of subjects are shown in Table 1. HIV-infected children showed significant lower NPT scores in closure, exclusion, memory, verbal meaning, quantity and hidden figure than control (Table 1). HIV-infected children showed significantly decreased ALFF in multiple brain sites including left middle temporal gyrus, left precentral and left post central gyrus as compared to control (Figure 1 and Table 2). The seed region in the left middle temporal gyrus of auditory language showed significantly reduced FC with clusters in the right inferior parietal and left postcentral in HIV-infected children. The seed region in the left precentral gyrus of motor network showed significantly reduced FC with cluster in the right vermis and significantly increased FC with cluster in the right precuneus and left middle frontal in HIV-infected children. The seed region in the left postcentral gyrus of sensory network showed significantly decreased FC with the cluster in the right middle temporal and significantly increased FC with cluster in the right superior parietal in HIV-infected children (Figure 1 and Table 2). Lower ALFF from the left middle temporal correlated with mazes, and reduced ALFF from the left post central correlated with closure and memory. The FC of seed region in the left precentral gyrus of motor network with right precuneus correlated with closure and memory, while FC with left middle frontal correlated with closure (Table 3).Discussion and Conclusion:
We observed significantly altered ALFF and FC in auditory, visual, language, motor and sensory networks in HIV-infected children. These changes were correlated with waning cognitive performance of HIV-infected children. The current findings suggest that the HIV affects the spontaneous-neural-activity and FC in pediatric patients, which may have significant impact on neurodevelopment and subsequently cognitive functioning.Acknowledgements
Sidra Medicine provides the work station to process the MRI data.References
1. Cohen S, Caan MW, Mutsaerts HJ, et al. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology 2016;86: 19-27. 2. Hoare J, Fouche JP, Spottiswoode B, et al. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naive "slow progressors". J Neurovirol 2012;18: 205-212. 3. Jankiewicz M, Holmes MJ, Taylor PA et al. White Matter Abnormalities in Children with HIV Infection and Exposure. Front Neuroanat 2017;11: 88. 4. Yadav SK, Gupta RK, Garg RK, et al. Altered structural brain changes and neurocognitive performance in pediatric HIV. Neuroimage Clin 2017;14: 316-322. 5. Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 2011;6: e25031.Figures
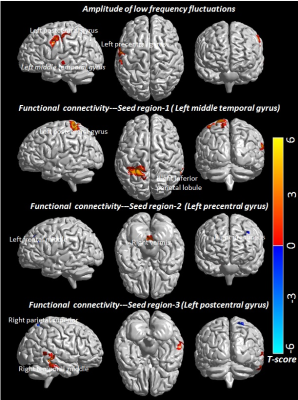
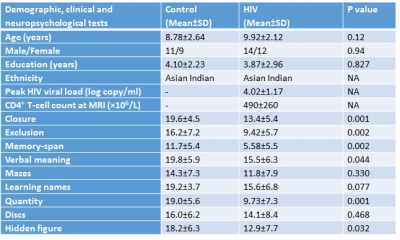
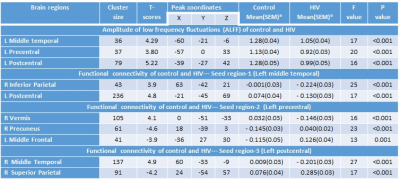
Table 2: Amplitude of low frequency fluctuations and functional connectivity in control and children with HIV. For each cluster, the coordinates of the peak difference as well as the anatomical location and size (in voxels) are shown.
L, Left; R, Right; SEM, Standard error of mean; *Means adjusted for age and sex.
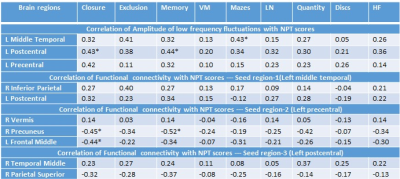
Table 3: Pearson correlation coefficients of amplitude of low frequency fluctuations and functional connectivity with neuropsychological test scores.
L, Left; R, Right; VM, verbal meaning, LN, learning name; HF, hidden figure; Neuropsychological tests, NPT; *significant at 0.005 level.