5432
Influences of the Gut Microbiome on Early Brain Development1Waisman Center, University of Wisconsin Madison, Madison, WI, United States, 2Psychology, University of Wisconsin Madison, Madison, WI, United States, 3Medicine, University of Wisconsin Madison, Madison, WI, United States, 4Psychiatry, University of Wisconsin Madison, Madison, WI, United States, 5Medical Physics, University of Wisconsin Madison, Madison, WI, United States
Synopsis
Increasing evidence from animal studies suggests the gut microbiome has a significant role on early brain development and function. However, little is known about this role on human brain development and in particular, on myelination. Using quantitative multicomponent relaxometry and 16S rRNA sequencing, we examined measures of myelin content and the gut microbiome from a cohort of typical developing infants. Infant brain measures were found to be differentially associated with the relative abundancies of certain bacteria phylum, suggesting that microbial communities may have a significant influence on processes of early brain development.
Introduction
The human brain undergoes rapid maturation that is guided by a complex interplay of genetics and environmental factors. In particular, there exists a compelling temporal overlap between the rapid colonization of microbiota and the development of the brain, suggesting a critical interaction between these two processes1. Increasing evidence from animal studies suggests that the gut microbiota has a substantial role on underlying brain mechanisms, including metabolism, inflammation, synaptogenesis, and myelination2-4, however, little evidence in the human exists. Recently, gut microbiota populations were found to be associated with cerebrospinal fluid (CSF) biomarkers of Alzheimer’s disease5, while a separate study found microbial compositions to be related to childhood cognition6. Levels of myelin content have also been shown to be related to these processes7,8, raising the hypothesis that microbiota may influence human myelination. In this work, we combined measures from the mcDESPOT9 multicomponent relaxometry technique and high-throughput 16S rRNA sequencing that were acquired from a cohort of typically developing infants to examine the relationships between myelin content and infant gut microbiota.Methods
MRI Acquisition: Twenty-four (13 female) typically developing infants (4.8 ± 1.44 months, corrected for gestation) were imaged using a 32-channel head RF coil on a GE MR750 3T scanner during non-sedated sleep. Multi-flip angle SPGR and bSSFP images were acquired and three-pool mcDESPOT post-processing9 was used to calculate parameter maps of the myelin water fraction (VFM), longitudinal (T1) and transverse (T2) relaxation times. Advanced Normalization Tools (ANTs)10 was used to create and normalize individual datasets to a study-specific template. Microbiota Characterization: Stool samples from each of the enrolled infants were collected within a week of the MRI scan. Bacterial 16S rRNA sequencing was performed by available services at the local institution, and the Qiime processing pipeline was used to characterize the taxonomy of the gut microbiota. Statistical Analysis: Relationships between the relative abundancies of gut microbiota phyla and VFM, T1, and T2 were performed using voxelwise statistics. Non-parametric permutation testing was performed using FSL’s randomise tool with 5000 permutations. Threshold-free cluster enhancement was utilized for correction of multiple comparisons.Results
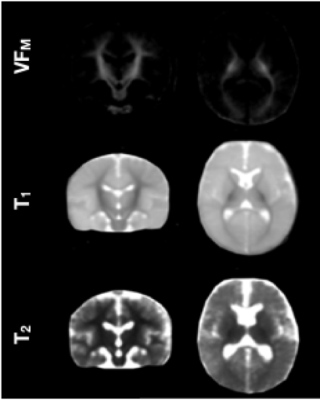
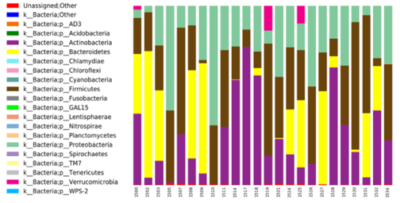
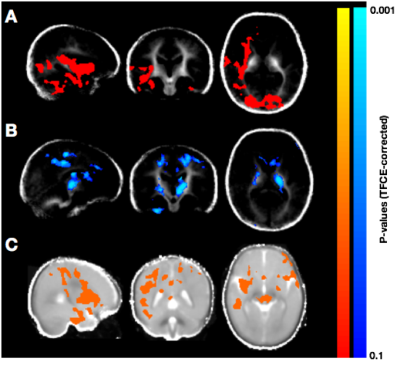
Population averaged VFM, T1, and T2 maps are shown in Fig. 1, The gut microbiome was heterogeneous and varied across individuals (Fig. 2). Of the different bacteria phyla present in the infant gut microbiome, Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes made up approximately 99.5% of all bacteria phylum for both males and females. We therefore restricted the analyses to these phyla. Positive associations between VFM and the relative abundancy of Firmicutes were observed across widespread brain regions, including the superior longitudinal fasciculus, optic radiations, and temporal white matter tracts (Fig. 3a). These relations did not withstand multiple comparison correction, however, a trend level (p<0.1) association was observed. We also found negative associations between VFM and relative abundancy of Proteobacteria across early developing brain regions, including the internal capsules and parietal white matter (Fig. 3b). Increased T1 was associated with the relative abundancy of Proteobacteria, though these relations remained at trend level after multiple comparison correction (Fig. 3c). We did not find any relations between T2 and any of the phyla relative abundancies.Discussion
These observed associations suggest that certain phyla may differentially influence early brain development, and in particular, myelination. In animal models, Firmicutes have been reportedly involved in energy resorption and metabolism11. Such processes are likely to be essential for brain development and myelination; thus, these bacteria may have an underlying role in these processes. Proteobacteria, on the other hand, have been implicated in inflammatory mechanisms and a compromised ability to maintain a balanced gut microbial community12. Hence, negative associations with VFM (and positive associations with T1) may indicate that an increased abundancy of the Proteobacteria phyla may disrupt processes of neurodevelopment.Results
In this work, we investigated whether the infant gut microbiome was associated with measures of brain myelination. We show, for the first time, that the relative abundancies of Firmicutes and Proteobacteria have a differential association on infant myelin content, as measured by VFM and T1. This presented work provides an important step for understanding the relationships between early brain maturation and the development of the gut microbiome. Future analyses will explore longitudinal relationships of infant brain and microbiome development as well as how these processes influence the cognitive function.Acknowledgements
We’d like all of the participants and their families. We’d also like to thank Joqauin Villaruz, Megan Lucas, Devin Ketelboeter, Janna Swearingen, Rishav Banerjee, and Michael Dean for their help in acquisition of the data. This work was supported by a Gates Foundation Grand Challenges Award (OPP1128547)References
1. Yang I, Corwin EJ, Brennan PA, Jordan S, Murphy JR, Dunlop A. The Infant Microbiome: Implications for Infant Health and Neurocognitive Development. Nurs Res 2016;65:76–88.
2. Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 2016;6:e774
3. Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology 2014;146:1525–33
4. Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol Stress 2016;4:23–33
5. Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep 2017;7:13537.
6. Carlson A, Xia K, Azcarate-Peril A, Goldman B, Ahn M, Styner M, Thompson A, Geng X, Gilmore J, Knickmeyer R. Infant Gut Microbiome Associated with Cognitive Development. Biol Psychiat 2017
7. Dean DC, Hurley SA, Kecskemeti SR, et al. Association of Amyloid Pathology With Myelin Alteration in Preclinical Alzheimer Disease. JAMA Neurology 74:41–49\
8. Deoni SC, O’Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N, Dirks H, Piryatinsky I, III DC, Jumbe NL. White matter maturation profiles through early childhood predict general cognitive ability. Brain structure & function:1–15
9. Deoni SC, Matthews L, Kolind SH. One component? Two components? Three? The effect of including a nonexchanging “free” water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magnetic Resonance in Medicine 70:147–154
10. Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical image analysis 12:26–41.
11. Krajmalnik-Brown R, Ilhan Z-EE, Kang D-WW, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract 2012;27:201–14
12. Shin N-RR, Whon TW, Bae J-WW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503
Figures