5430
In vivo 1H NMR spectroscopy study of mitochondrial pyruvate carrier 1 (MPC1) deficient mouse reveals energy shift in the development of embryonic brain1Center for Biomedical Imaging (CIBM-AIT), École Polytechnique Fédérale de Lausanne, Lasuanne, Switzerland, 2University of Geneva, Geneva, Switzerland
Synopsis
Pyruvate degradation is an important step for oxidative phosphorylation. We studied brains of murine embryos devoid of mitochondrial pyruvate carrier 1 (MPC1) using 1H MRS. We demonstrates that after the embryonic energy shift, lactate accumulation can explain embryonic lethality in embryo devoid of MPC1.
Introduction
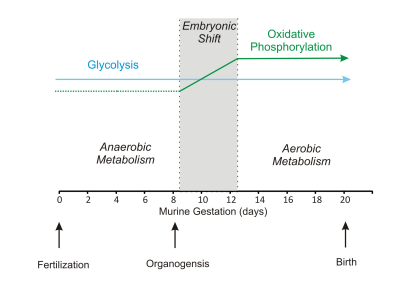
Pyruvate degradation is an important step for oxidative phosphorylation. Mitochondrial pyruvate carrier 1 , (MPC1) is a central step which links cytosolic and mitochondrial intermediary metabolism and can regulate the metabolic state necessary for embryonic development. In this study, we investigated whether quality MR images can be obtained from E12.5 (the end of embryonic shift, Figure 1, 1) to E18.5 (close to birth, Figure 1). Secondly, we studied abundant metabolites in embryonic brain of mice at E12.5 when oxidative phosphorylation in mitochondrial severs for proper embryonic and fetal development. Furthermore, we studied mice deficient with mitochondrial pyruvate carrier 1 (MPC1) at E12.5, when lethality in embryo devoid of MPC1 occurs thereafter (2).Methods
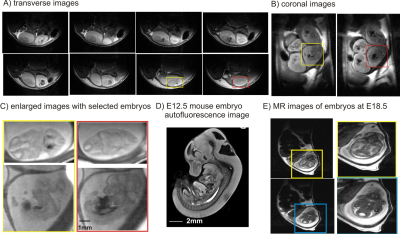
All animal study was performed according to the local ethical guidelines for in vivo experimentation. Two pairs of female and male mice were used in this study. One pair of mice bearing the MPC1gt allele (2) underwent timed mating at the end of the day and the presence of a vaginal plug was checked the following morning. This time was taken as E0.5. Since embryonic lethality occurs approximately E13-13.5 in MPC1 deficient mice, we terminated the study right after E12.5 on the pregnant mouse for genotype (2). Another pair without the MPC1gt allele underwent the identical timed mating procedures and the pregnant mouse was studied at E18.5. All measurements were performed at 14T. A quadrature coil (two geometrically decoupled 16-mm-diameter loops) was used for radio-frequency transmitting and receiving. The pregnant animals were lying on the surface coil with one side. Throughout the entire study, animals were kept anesthetized under 1.5-2% isoflurane mixed with air and oxygen (1:1) through a mask to maintain their respiration rates within the range of 80-100 beats-per-minute. Their body temperatures were monitored (SA Instruments. U.S.A.) and well-maintained at 36-37°C by circulating warm water via silicon tubes. Anatomical images were acquired with fastest spin echo (TEeff/TR=50/4000ms, 4 averages). STEAM (TE/TM/TR=2.8/20/4000ms, 3) with outer volume suppression and water suppression was used for localized 1H MRS. LcModel (4) was applied to analyze spectral data referencing to the endogenous water (90%, 5).Results and Discussion
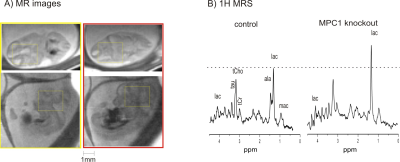
Quality images were obtained with sufficient resolution to illustrate embryonic brain (Figure 2). Such images depicted anatomical evolution of embryonic brain development and allowed locating the region of interested for localized 1H MRS (Figure 3A). In the pregnant mouse bearing MPC1gt, seven embryos were observed in anatomical images and six of them were measured using 1H MRS. Amongst, two embryos devoid of MPC1 (MPC1 knockout or MPC1-/-) and the rest four embryos were homozygous and heterozygous with MPC1(controls). Noticeably, lactate was elevated in embryonic brains devoid of MPC1 (Figure 3B). Further quantification confirmed our observation, i.e. 22.3±2.2 vs. 36.6 ±1.9 μmol/g(controls vs. knockout mice, two-way ANOVA on genotype and metabolite factors, p<0.0001). The accumulation of lactate in embryonic brain devoid of MPC1 at E12.5 reinforces the hypothesis that MPC1 is a central step which links cytosolic and mitochondrial intermediary metabolism and a key player in regulating the metabolic state necessary for embryonic development.
In summary, we demonstrate that 1H MRS of embryonic brain at E12.5 is feasible on mouse. The in vivo results allowed better characterizing the underling mechanism of lethality in MPC1 deficient embryos.
Acknowledgements
This work was supported by the Centre d'Imagerie BioMédicale of the UNIL, UNIGE, HUG, CHUV, and EPFL and the Leenaards, Jeantet and Gianni Biaggi de Blasys Foundations.References
1. Baker CN and Ebert SN Development of aerobic metabolism in utero: Requirement for mitochondrial function during embryonic and fetal periods. OA Biotechnology 2013 Apr 01;2(2):16.
2. Vanderperre B, Herzig S, Krznar P, Hörl M, Ammar Z, Montessuit S, et al. (2016) Embryonic Lethality of Mitochondrial Pyruvate Carrier 1 Deficient Mouse Can Be Rescued by a Ketogenic Diet. PLoS Genet12(5): e1006056.
3. Tkáč, I., Starčuk, Z., Choi, I.-Y. and Gruetter, R. (1999), In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med, 41: 649–656.
4. Provencher, S. W. (1993), Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med, 30: 672–679.
5. Katzman, R., and Pappius, H. (1973). Brain Electrolytes and Fluid Metabolism, Williams & Wilkins, Baltimore, pp. 1–13
Figures