5428
Late post-natal brain metabolic changes in healthy rats, a longitudinal in vivo 1H and 31P MRS study1Laboratory of Functional and Metabolic Imaging (LIFMET), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 2Neurometabolic Unit, Service of Clinical Chemistry, University Hospital of Lausanne, Lausanne, Switzerland, 3Centre d’Imagerie Biomédicale (CIBM), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 4Swiss Center for Liver Disease in Children, University Hospitals Geneva, Geneva, Switzerland
Synopsis
New-born rats are known to undergo important brain metabolic changes during first 4-5 weeks of their lives. However precise description of metabolic changes at latter stages is missing. At this period rats might still undergo changes due to final brain maturation what may impact data interpretation of experiments during this window. This study aimed to explore possible metabolic changes in the rat brain during the late post-natal period (post-natal days 29 - 77), using in-vivo 1H and 31P-MRS and a temporal resolution of 1 week. We report significant changes in Ins, tCho (mainly due to GPC increase), Tau and Glu in rats considered as young adults.
Introduction
New-born rats undergo many brain metabolic changes (NAA, neurotransmitters, creatine and phosphocreatine, antioxidants, taurine, myo-inositol and metabolites involved in phospholipid metabolism) due to maturation of brain structures and functions1–6.
Most of these changes were reported with high temporal resolution only until post-natal day 28 (p28) or p351–6. Although rats are accepted to have reached adulthood after p28, they still might undergo some metabolic changes linked with the final maturation of the brain (e.g.myelination). To date there are no detailed descriptions of the metabolic changes during this later maturation phase in early adulthood. These possibly present changes may impact data interpretation of experiments during this window of time.
The aim of this study was to explore possible metabolic changes in the rat brain during the late post-natal period (p29-p77), using in vivo 1H and 31P-MRS and a temporal resolution of 1 week.
Methods
1H-MRS longitudinal measurements were performed on two groups of rats (male, Wistar) that were interleaved in order to decrease the exposure of each animal to anesthesia: 8 rats were scanned on p29 and every two weeks thereafter until p71; 8 other rats were scanned at p35 and every two weeks thereafter until p77. Therefore a total of 8 measurements were obtained for each weekly time-point between p29-p77. 31P-MRS spectra were acquired on 4 rats at p35, p49 and p63.
Experiments were performed on 9.4T-system (Varian/Magnex Scientific) using home-built coils (1H-MRS: quadrature 1H-surface coil; 31P-MRS: quadrature 1H-loops with single 31P-loop) and FASTMAP7 for shimming. 1H-MRS spectra were acquired in hippocampus (2×2.8×2mm3) using SPECIAL8 (TE/TR=2.8ms/4s,160avg.) and concentrations of metabolites were calculated by LCModel using water as reference. 31P-MRS spectra were acquired using a non-selective AHP pulse for excitation, localized by OVS(x,z) and 1D-ISIS(y)(TR=8s,384avg.), WALTZ-16 for NOE and 1H-decoupling in a VOI=5×9×9mm3. 31P-MR spectra were quantified by AMARES (jMRUI)9 and normalized for each rat using its PCr concentration from 1H-MRS in the same VOI.
Results
Fig1 shows evolution of brain metabolites between p29-p77 measured by 1H-MRS. As expected, no further changes were measured for most metabolites after p29. However, a significant continuous increase between p29-p77 was observed for Ins (+33±9%) and tCho (+53±12%). Glu showed a slight increase (+10±6%) until p43 and remained constant thereafter. Tau concentration dropped between p29 and p43 by 12±6% and decreased slowly by an additional 8±12% until p77. Lac showed also some variations without reaching statistical significance. We did not observe any significant change in NAA, Cr or PCr. Fig2 shows evolution of brain metabolites between p35-63 measured by 31P-MRS. Only GPC showed a significant increase of 67±25%, in agreement with the increase of tCho (PCho+GPC) measured by 1H-MRS. Fig3 shows the quality of 1H and 31P-MRS spectra with visible increase in Ins, tCho and GPC.Discussion and Conclusions
Present data report significant changes in Ins, tCho (mainly due to GPC increase), Tau and Glu in late post-natal period.
Ins was shown to increase from birth until p281, thus the increase observed in our study is in agreement, showing a continuous change which might be due to ongoing myelination.
The increase in tCho was confirmed by the significant increase in GPC measured by 31P-MRS(Fig2). tCho is known to decrease between p7-281; the sudden increase observed at our later time points might be explained by changes in enzymatic activities around p28, as phospholipids are known to undergo intense metabolism during development. In addition, Huxtable et al.4 showed sharp decrease of phosphatidyl-choline and phosphatidyl-ethanolamine (phospholipids,unmeasurable by MRS) in synaptosomes until p28 with a slight increase between p28 and p56. This switch of phospholipid content in synaptosomes is in line with: (1)decrease in PE and tCho observed from birth until p281 and (2)constant PE and increasing tCho (constant PCho and increase in GPC) from p29 to p77 observed in this study.
Tau is well known to decrease postnatally1,4,6. According to our data, Tau stopped its rapid decrease around p35-42 and afterwards continued to decrease slowly until the end of the study at p77. Some studies already showed that Tau decreased between p28 and adulthood4,6 but without additional measurements between these two time-points.
Gln and Glu were both shown to increase between birth and p281,5, probably due to maturation of neurotransmitter processes and enzymes involved in amino-acid metabolism. Our study showed that Glu continues to increase until p43 whereas Gln stayed stable from p29.
In contrast to changes in Tau and Glu that reached a plateau within the time frame of our study, Ins and tCho increased significantly until p77. Therefore it would be interesting to determine the exact period of stabilization of these changes.
Acknowledgements
Supported by CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards and Jeantet Foundations. EU: FP7-PEOPLE-2012-ITN project 316679 TRANSACT. The SNSF project no 310030_173222/1.References
1. Tkác I, Rao R, Georgieff MK, Gruetter R. Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med [Internet]. 2003 Jul [cited 2013 Oct 3];50(1):24–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12815675
2. Terpstra M, Rao R, Tkac I. Region-specific changes in ascorbate concentration during rat brain development quantified by in vivo 1H NMR spectroscopy. NMR Biomed. 2010;23(9):1038–43.
3. Burri R, Bigler P, Straehl P, Posse S, Colombo J-P, Herschkowitz N. Brain development: 1H magnetic resonance spectroscopy of rat brain extracts compared with chromatographic methods. Unbekannt. 1990;15(10):1009–16.
4. Huxtable RJ, Crosswell S, Parker D. Phospholipid composition and taurine content of synaptosomes in developing rat brain. Neurochem Int. 1989;15(2):233–8.
5. Bayer SM, McMurray WC. The Metabolism of Amino Acids in Developing Rat Brain. J Neurochem. 1967;14:695–706.
6. Agrawal HC, Davison AN, Kaczmarek LK. Subcellular distribution of taurine and cysteinesulphinate decarboxylase in developing rat brain. Biochem J. 1971;122(5):759–63.
7. Gruetter R, Tkác I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med [Internet]. 2000 Feb;43(2):319–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10680699
8. Mlynárik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med [Internet]. 2006 Nov [cited 2013 Nov 10];56(5):965–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16991116
9. (http://www.mrui.uab.es/mrui)
Figures
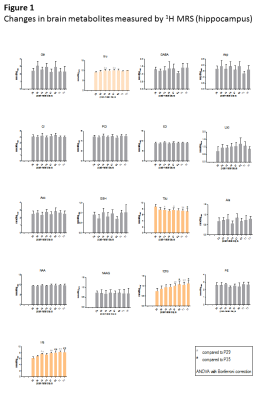
The only metabolites that showed significant change between p29-p77 were Glu, Tau, tCho and Ins (histograms in orange).
ANOVA with Bonferroni correction was performed on each group of animals separately (group1: p29,p43,p57 and p71; group2: p35,p49,p63 and p77) as there are some variations in metabolites as an effect of group due to inter-animal variability (as seen on Gln or Asp).
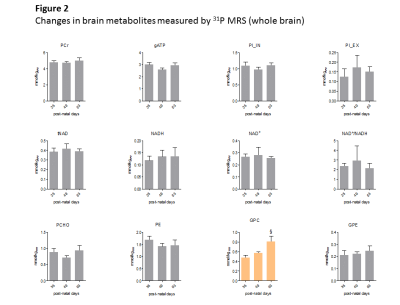
The only metabolite that showed significant change between p35-p63 was GPC (histogram in orange).
PCr measured by 1H MRS in the same VOI was used as concentration reference. Student’s t-test was used for statistics.
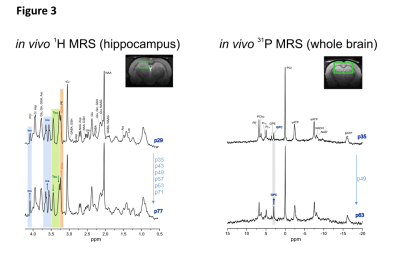
Left: 1H MRS spectra acquired in hippocampus (2x2.8x2mm3) at p29 (first time-point) and p77 (last time-point) showed visible increase in Ins (blue) and tCho (orange) peaks and decrease in Tau (green) peaks. Both spectra are from the same animal.
Right: 31P MRS spectra acquired in big voxel (5x9x9mm3) at p35 (first time-point for 31P MRS) and p63 (last time-point) showed visible increase in GPC. Both spectra are from the same animal.