5425
The Impact of Prenatal Endocrine-Disrupting-Chemical Exposure on Brain Function in Teenagers1Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung, Taiwan, 2National Institute of Environmental Health Sciences, National Health Research Institutes, Miaoli, Taiwan, 3Institute of Medicine and Department of Medical Imaging, Chung Shan Medical University and Hospital, Taichung, Taiwan, 4School of Medicine and Department of Pediatrics, Chung Shan Medical University and Hospital, Taichung, Taiwan, 5Department of Medical Imaging and Radiological Sciences, Chang Gung University, Taoyuan, Taiwan, 6Department of Psychiatry, Chang Gung Memorial Hospital, Chiayi, Taiwan
Synopsis
The products made by endocrine disrupting chemicals (EDCs) widely exists in our daily life, which could be a risk of our health. We tried to find the relationship between prenatal exposure to EDCs’ concentrations and teenager’s brain function using resting-state functional MRI (rs-fMRI). Our results showed the correlation between prenatal perfluorononanoic acid (PFNA)/MeHg exposure and functional alterations of the teenage brain in caudate gyrus and putamen.
Introduction
The perfluorochemicals (PFCs) and methylmercury (MeHg) are widespread and constant in the environment and belong to endocrine disrupting chemicals (EDCs). Several studies reported PFCs and MeHg could be absorbed by the human from seafood and meat 1,2. Some studies showed the correlation between prenatal exposure to PFCs or MeHg and decreased IQ test scores in children 3,4. However, the effect of PFCs and MeHg on brain function is still unknown. Therefore, our study aimed to find out the relationship between prenatal exposure to PFCs/MeHg and teenage brain function with resting-state functional MRI (rs-fMRI).Methods
We recruited 59 mother-child pairs from the general population (33 male, 26 female) and examined the association between PFCs/MeHg in the maternal serum collected during the third trimester of pregnancy and the teenager's brain MRI at 13 to 16 years of age (mean ± SD = 13.95 ± 0.47). The PFCs including perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), perfluorononanoic acid (PFNA), perfluoroundecanoate acid (PFUnDA), and perfluorododecanoic Acid (PFDoDA).
All functional MR images were acquired using a 3-Tesla MRI (Skyra, Siemens, Germany) with a 20-channel head-neck coil. Images were acquired with the following parameters: TR = 2000 ms, TE = 30 ms, voxel size= 2.7 × 2.7 × 4 mm3, number of scan = 240, axial slice = 28. The resting-state fMRI raw data were processed with Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK) and Resting-State fMRI Data Analysis Toolkit (REST1.8, Lab of Cognitive Neuroscience and Learning, Beijing Normal University, China). In preprocessing, the images were corrected for the differences in slice acquisition times and head motion, and the corrected images were spatial normalized to Montreal Neurological Institute (MNI) template (each voxel was resampled to 3×3×3 mm3). The normalized images were smoothed with a Gaussian kernel of the 6 mm full-width at half maximum (FWHM = 6 mm). No smoothing was performed in mean regional homogeneity analysis. The linear trend of the functional data was then removed, and a band filter with 0.01-0.12 Hz was applied. After removing physiological noises, mean fractional amplitude of low-frequency fluctuation (mfALFF) and mean regional homogeneity (mReHo) was performed to calculate resting-state brain activity, and the correlation between the maternal PFCs/MeHg concentrations and the teenage brain MRI were estimated using multiple regression analysis by SPM8 with adjustments for the teenage sex and family income. In addition, we also divided our data into boy and girl groups for comparison.
Results
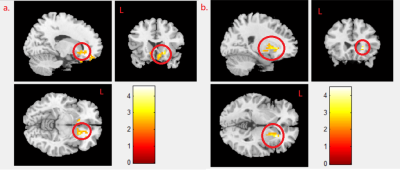
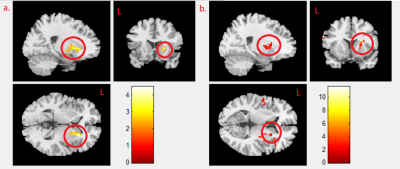
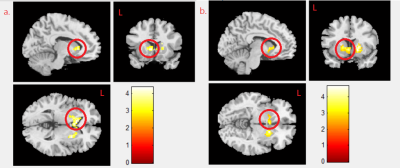
We found negative correlation between MeHg concentration and mfALFF activity in the right caudate gyrus in boy and girl combined group (p<0.02) (Fig. 1a), and we also found the negative correlation between MeHg concentration and mReHo in the caudate in the combined group (p<0.021) (Fig. 1b). A negative correlation between MeHg concentration and mReHo was found in the right putamen in combined group (p<0.021) (Fig. 2a), and the same finding was shown in girl group (p<0.04) (Fig. 2b). We observed a negative correlation between PFNA concentration and mReHo in the left caudate gyrus in combined group (p<0.004) (Fig.3a) as well as in boy group (p<0.009) (Fig. 3b).Discussion
Previous studies showed prenatal exposure to MeHg could affect working memory in children 5, and the reward system in the brain could indirectly affect working memory 6,7. The putamen and caudate involved reward system in the brain by modulated the dopamine release directly 8,9, therefore based on our findings we suggested that these region dysfunctions lead to adverse effect of working memory by prenatal exposure to MeHg. The pervious study reported the maternal PFNA concentration was related to attention deficit hyperactivity disorder (ADHD) symptoms in early childhood 10, and several studies showed asymmetric caudate gyrus volume in ADHD patient 11-13, which were consistent with our results. ADHD is more common in boys 14,15, and it might be the reason for our result only showed in boy group.Conclusion
Our results provided the evidence of the relationship between prenatal PFNA/MeHg exposure and functional alterations of the teenage brain in caudate gyrus and putamen, and may be an important public health issue.Acknowledgements
This study was supported in part by the nationwide Taiwan Maternal and Infant Cohort Study, and Grant from Taiwan NHRI (Nat'l Health Res Ins): EM-105-PP-05, EM-105-SP-16, EO-104-SP-01.References
1. Thorhallur IH, Chunyuan F, J Ørn O et al. Dietary Predictors of Perfluorinated Chemicals: A Study from the Danish National Birth Cohort. Environ. Sci. Technol. 2008; 42(23): 8971-77.
2. Line EK, Jan SJ, Flemming N et al. Public health benefits of hair-mercury analysis and dietary advice in lowering methylmercury exposure in pregnant women. Scandinavian Journal of Public Health 2017; 45: 444–451.
3. Yan W, Walter JR, Hsin-Yi C et al. Prenatal exposure to perfluroalkyl substances and children’s IQ: The Taiwan maternal and infant cohort study. International Journal of Hygiene and Environmental Health 2015; 218: 639–644.
4. Joseph LJ, Gina M, Pierre A et al. Relation of Prenatal Methylmercury Exposure from Environmental Sources to Childhood IQ. Environmental Health Perspectives 2015; 123(8): 827-833.
5. Olivier B, Gina M, Joseph LJ et al. Domain-Specific Effects of Prenatal Exposure to PCBs, Mercury, and Lead on Infant Cognition: Results from the Environmental Contaminants and Child Development Study in Nunavik. Environmental Health Perspectives 2014; 122(3): 310-316.
6. Jessica AG, John AP, Adrian MO, The role of the basal ganglia in learning and memory: Neuropsychological studies. Behavioural Brain Research (2009); 199: 53–60.
7. Yen Y, Thomas HBF, Karl JF, Working Memory and Anticipatory Set Modulate Midbrain and Putamen Activity. The Journal of Neuroscience (2013); 33(35):14040-14047.
8. Aaron JG, Peter D, Boris SG et al. Dopamine modulation in the basal ganglia locks the gate to working memory. J Comput Neurosci (2006); 20: 153–166.
9. Petter M, Anne L, Eva E, Temporal dynamics of basal ganglia under-recruitment in Parkinson’s disease: transient caudate abnormalities during updating of working memory. Brain (2009); 132: 336-346.
10. Guang-Wen L, Ching-Chun H, Jia-Shian S et al. Perfluoroalkyl substances in cord blood and attention deficit/hyperactivity disorder symptoms in seven-year-old children. Chemosphere (2016); 156: 118-127.
11. Luis GAM, Josefina R, Lázaro BDLT et al. Clinical correlations of grey matter reductions in the caudate nucleus of adults with attention deficit hyperactivity disorder. Psychiatry Neurosci (2010); 35(4): 238-246.
12. Gregory WS, Rebecca LB, Edward FJ et al. Caudate Nucleus Volume Asymmetry Predicts Attention-Deficit Hyperactivity Disorder (ADHD) Symptomatology in Children. J Child Neurol (2002); 17: 877–884.
13. Linh CD, Gregory RS, Jacob SY et al. Caudate asymmetry is related to attentional impulsivity and an objective measure of ADHD-like attentional problems in healthy adults. Brain Struct Funct. (2016); 221(1): 277–286.
14. Steven PC, Charity GM, Robert EM. Prevalence and Correlates of ADHD Symptoms in the National Health Interview Survey. Journal of Attention Disorders (2005); 9(2): 392-401.
15. F. Xavier C, Jay NG, Paul E et al. Quantitative Morphology of the Caudate Nucleus in Attention Deficit Hyperactivity Disorder. Am J Psychiatry. (1994); 151(12): 1791-6.
Figures