5423
Fetal T1 mapping using multi-inversion EPI at 3T1Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children's Hospital, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Neuroscience and Biomedical Engineering, Aalto University School of Science, Espoo, Finland, 5Department of Newborn Medicine, Harvard Medical School, Boston, MA, United States, 6Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 7Harvard-MIT Health Sciences and Technology, Institute of Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 8Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
T1 mapping of the fetal brain is hindered by substantial, frequent, and random fetal motion, making many current quantitative techniques impractical in the fetus. We have applied to the fetal brain (and adult brain for validation purposes) a recently introduced T1 mapping technique based on EPI readout that is preceded by a non-selective inversion pulse, and where the order of the acquired slices is permuted from one inversion recovery to the next, allowing efficient, high temporal sampling of the T1 relaxation curve. We believe that this method is capable of providing accurate T1 maps of the fetal brain.
Introduction/Purpose
Fetal imaging is hindered by unpredictable motion of the fetus preventing robust and reliable fetal MRI by current techniques. Thus, fetal imaging in general is limited to fast, single-shot 2D encoding acquisitions [1]. Consequently, to date, robust and accurate quantification of tissue relaxivity (T1, T2) in fetal brain or body remains an unsolved problem, in spite of previous initial attempts using magnetic resonance fingerprinting (MRF) [2,3].
Multi-inversion (MI) EPI acquisition methods [4-7], allow rapid sampling of the longitudinal magnetization recovery. This is achieved by playing a non-selective inversion pulse followed by a 2D multi-slice EPI readout, such that the acquisition order of the slices is permuted, so each slice is experiencing a different effective inversion time (TI) within each recovery period. The purpose of this study is to employ the MI-EPI acquisition method [4,5] to fetal imaging, and test its feasibility in obtaining quantitative T1 estimates in the fetal brain.
Methods
Four fetal and one adult (for validation purposes) brains were scanned on a 3T scanner (Skyra, Siemens Healthcare), and all participants signed IRB approved informed consent forms. Our MI-EPI fetal acquisition had these specifics: FOV=320mm, matrix size=160×160, 2mm thick slices, GRAPPA R=2, TR=5.25s, TE=32ms. With 49 slices, 49 measurements, and skip factor of 7 [4,5], TAacq=4:28min. This yielded seven sets of volumes, each sampling the 7 points along the magnetization recovery curve with TI ranging from 24ms to 4298ms. The inversion was performed using an adiabatic FOCI pulse [8].
The acquired images were visually inspected, and if considerable motion was observed in more than half of the volumes, the T1 maps were labeled as unreliable. For datasets where the fetus remained mostly still, only volumes with severe motion were discarded from further processing. Matlab scripts were then used to model each voxel in each slice with a mono-exponential T1 recovery including a lag term to compensate for incomplete T1 recovery. Voxels that failed this modeling were identified and filtered by a 3-dimensional 26-neighborhood median.
Results
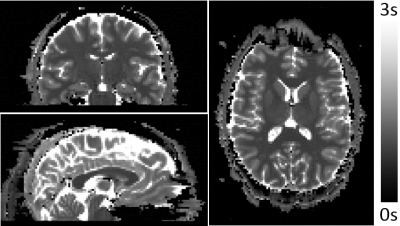
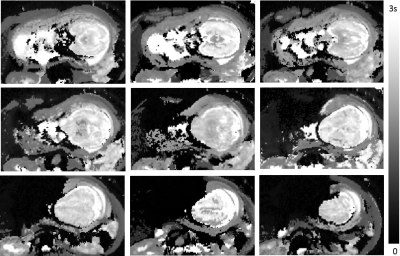
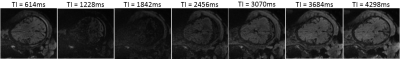
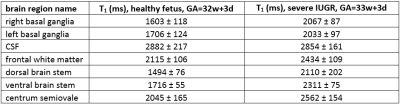
Figure 1 shows estimated T1 maps of the healthy adult, yielding average T1 values of ~820ms and ~1500ms in the white and gray matter, respectively, consistent with many prior studies. Two fetal scans resulted in unreliable fetal brain T1 maps due to severe fetal motion. In the remaining two, fetal motion was limited, and T1 maps appeared reasonable. Figure 2 shows estimated T1 maps of a 33w+3d old fetus diagnosed with severe Intrauterine Growth Restriction (IUGR). Figure 3 shows one slice of the same MI-EPI acquisition across 7 volumes, demonstrating the contrast modulation achieved by permuting the order of the slices each inversion period. Table 1 presents the mean and standard deviation of the estimated T1 values in 7 different brain regions for the abovementioned IUGR fetus and the other 32w+3d (typically developing) fetus.Discussion/Future Work
Our current method is capable of obtaining reasonable quantitative fetal brain T1 maps. Fetal motion and maternal breathing still impose challenges, which are difficult to mitigate using state-of-the-art fetal BOLD-fMRI registration techniques [11,12], because of the temporally varying image contrast caused by the slice permutation. The fetal T1 maps however, provide expected regional anatomical contrast, and T1 values that are within the range previously reported for premature neonates [9,10]. While T1 values for CSF were almost identical between the two fetuses, the IUGR fetus had consistently longer T1 values in all brain regions compared to the 1 week younger typically developing fetus. This raises the possibility that regional T1 values may allow quantitative monitoring of brain development and detection of altered brain maturation. However, both larger prospective study, as well as additional pulse sequence development are needed to validate these preliminary findings.
Future improvements will include: 1. Adding low resolution 3D-EPI navigator at the end of the TR to estimate fetal motion for each volume, similarly to fetal HASTE-vNav imaging [13]; 2. Using the obtained fetal brain T1 estimates to optimize imaging protocol timing (currently optimized for adult brain); 3. Ensuring that full inversion is achieved in the pregnant abdomen by B1+/B1- mapping; 4. Extending the current method to asymmetric spin-echo EPI readouts for simultaneous T1/T2/T2’ fetal brain mapping.
Conclusion
We demonstrated, for the first time, the feasibility of the MI-EPI acquisition method for quantitative T1 fetal brain mapping. Due to the inherent acquisition speed of 2D-EPI readouts and the time-efficient sampling of the magnetization curve, this method is promising for obtaining brain T1 maps in the moving fetus. Nevertheless, at this point, fetal motion still hinders the accuracy and robustness of the method, which drives our proposals for future work.Acknowledgements
NIH grants R01-EB017337, R01-EB019437, U01-HD087211, R01-EB008547, R01-HD071664, P41 EB015902, R00-HD074649, R21-AG046657 and R01-HD071664References
1. Gholipour A, Estroff JA, Barnewolt CE, Robertson RL, Grant PE, Gagoski B, Warfield SK, Afacan O, Connolly SA, Neil JJ, Wolfberg A, Mulkern RV. Fetal MRI: A Technical Update with Educational Aspirations. Concepts Magn Reson Part A Bridg Educ Res. 2014;43(6): 237-266.
2. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature. 2013; 495(7440): 187-92
3. Gagoski B, Ye H, Cauley S, Bhat H, Setsompop K, Chatnuntawech I, Martin A, Jiang Y, Griswold M, Adalsteinsson E, Grant PE, Wald LL. Magnetic resonance fingerprinting for fetal imaging at 3T - initial results. Proc. Int. Soc. Magn. Reson. Med. 2015; p5212.
4. Renvall V, Witzel T, Wald LL, Polimeni JR. Fast variable inversion recovery time EPI for anatomical reference and quantitative T1 mapping. Proc. Int. Soc. Magn. Reson. Med. 2014; 22: p4282.
5. Renvall V, Witzel T, Wald LL, Polimeni JR. Automatic cortical surface reconstruction of high-resolution T1 echo planar imaging data, Neuroimage. 2016; 134: 338–354.
6. Dougherty R, Mezer A, Zhu K, Kerr A, Middione M. Fast quantitative T1 mapping with simultaneous multi-slice EPI. In: Annu. Meet. Organ. Hum. Brain Mapp. 2002; p2014.
7. Grinstead JW, Wang D, Bhat H, Deshpande V, Cauley SF, Setsompop K, Benner T, Anderson VC, Rooney WD. Slice-accelerated inversion recovery T1 mapping. Proc. Int. Soc. Magn. Reson. Med. 2014; p3215.
8. Hurley AC, Al-Radaideh A, Bai L, Aickelin U, Coxon R, Glover P, Gowland PA. Tailored RF pulse for magnetization inversion at ultrahigh field. Magn Reson Med. 2010; 63(1):51-8.
9. Williams LA, Gelman N, Picot PA, Lee DS, Ewing JR, Han VK, Thompson RT. Neonatal Brain: Regional Variability of in Vivo MR Imaging Relaxation Rates at 3.0 T – Initial Experience. Radiology 235, 595–603 (2005).
10. Schneider J, Kober T, Bickle Graz M, Meuli R, Hüppi PS, Hagmann P, Truttmann AC. Evolution of T1 relaxation, ADC, and fractional anisotropy during early brain maturation: A serial imaging study on preterm infants. Am. J. Neuroradiol. 37, 155–162 (2016).
11. Luo J, Abaci Turk E, Bibbo C, Gagoski B, Roberts DJ, Vangel M, Tempany-Afdhal CM, Barnewolt C, Estroff J, Palanisamy A, Barth WH, Zera C, Malpica N, Golland P, Adalsteinsson E, Robinson JN, Grant PE. In Vivo Quantification of Placental Insufficiency by BOLD MRI: A Human Study. Sci. Rep. 7, 3713 (2017).
12. Turk EA, Luo J, Gagoski B, Pascau J, Bibbo C, Robinson JN, Grant PE, Adalsteinsson E, Golland P, Malpica N. Spatiotemporal alignment of in utero BOLD-MRI series. J Magn Reson Imaging. 2017; 46(2):403-412
13. Gagoski B, McDaniel P, van der Kouwe AJV, Bhat H, Wald LL, Adalsteinsson E, Grant PE, Tisdall MD, HASTE imaging with EPI volumetric navigators for real-time fetal head motion detection. Proc. Int. Soc. Magn. Reson. Med. 2016; 24: p4413.
Figures