5418
Multicentre pediatric brain tumour dynamic susceptibility contrast (DSC-) MRI with contrast agent leakage correctionStephanie Withey1,2,3, Jan Novak4, Lesley MacPherson5, Laurence Abernethy6, Barry Pizer7, Richard Grundy8, Simon Bailey9, Dipayan Mitra10, Theodoros Arvanitis2,3,11, Dorothee Auer12, Shivaram Avula6, and Andrew Peet2,3
1RRPPS, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom, 2Oncology, Birmingham Children's Hospital, Birmingham, United Kingdom, 3Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, United Kingdom, 4Aston University, Birmingham, United Kingdom, 5Radiology, Birmingham Children's Hospital, Birmingham, United Kingdom, 6Radiology, Alder Hey Children's NHS Foundation Trust, Liverpool, United Kingdom, 7Oncology, Alder Hey Children's NHS Foundation Trust, Liverpool, United Kingdom, 8The Children’s Brain Tumour Research Centre, University of Nottingham, Nottingham, United Kingdom, 9Sir James Spence Institute of Child Health, Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom, 10Neuroradiology, The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom, 11Institute of Digital Healthcare, University of Warwick, Coventry, United Kingdom, 12Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom
Synopsis
Dynamic susceptibility contrast (DSC-) MRI provides measures of relative cerebral blood volume (rCBV) in pediatric brain tumors. Correction of rCBV for the effects of contrast agent leakage can be done using post-processing techniques. Forty pediatric patients with high and low grade tumors underwent DSC-MRI scans pre-treatment at four centers. DSC-data was analyzed with and without leakage correction to calculate rCBV and the leakage parameter, K2. There were significant differences between all parameters when comparing the high and low grade tumor groups. Low grade tumors tended to show T1-dominant leakage effects while high grade tumors showed predominantly T2* dominance.
Introduction
Dynamic susceptibility contrast (DSC-) MRI provides measures of relative cerebral blood volume (rCBV) in pediatric brain tumours. High grade gliomas have been shown to have significantly higher rCBV than low grade gliomas1. Study numbers can be increased by looking at multicentre data, however DSC-MRI protocols vary between centres. Leakage of contrast agent can affect the accuracy of rCBV values but post-processing methods such as that set out by Boxerman2,3 can be used to correct this. The aim of this work was to investigate leakage-corrected rCBV values in multicentre pediatric data and compare them between low (LGT) and high grade brain tumours (HGT).Methods
Forty patients with HGTs (WHO grades III and IV) and LGTs (WHO grades I and II) underwent MRI scans at four different centers prior to undergoing treatment. Details of the scanners and DSC-MRI protocols used are shown in Table 1. 0.1 mmol/kg of gadolinium contrast agent was administered. A pre-bolus dose to minimize T1 effects was given at some centers. DSC-MRI data was analysed using the Boxerman method2,3 with the leakage correction parameter, K2, allowed to be both positive (T1-dominant leakage effects) and negative (T2*-dominant leakage effects). Pixel-by-pixel uncorrected and corrected rCBV were calculated as the area under the uncorrected and corrected concentration-time curves respectively. Regions-of-interest were defined around the whole tumor. Whole-tumor average uncorrected and corrected rCBV and K2 were calculated. A t-test was used to compare the parameters obtained in HGTs and LGTs.Results
Mean patient age was 8.5 (range 0.4 – 17.6) years old. HGTs comprised medulloblastoma (n = 14), anaplastic astrocytoma (n = 2), CNS PNET (n = 3), GBM (n = 2) and ependymoma (n = 4); LGTs comprised pilocytic astrocytoma (n = 12), DNET (n = 1), oligodendroglioma (n = 1) and atypical choroid plexus papilloma (n = 1). Image quality was variable between centers and protocols. Example T2-w images, uncorrected and corrected rCBV maps and K2 maps are shown for two cases (Figure 1). Table 2 shows mean and standard deviation uncorrected rCBV, corrected rCBV and K2. There were significant differences between all parameters when comparing the LGT and HGT groups although a large overlap between values was seen (Figure 2). LGTs tended to show T1-dominant effects while HGTs on average showed T2* dominance.Discussion
Ho et al.1 reported significant differences between uncorrected rCBV in pediatric low and high grade gliomas. Later studies4,5 show increases in observed signal intensity over baseline –T1-dominant leakage - in pediatric low grade gliomas while T2*-dominant behavior was observed in high grade gliomas. This was not quantified. Pediatric low grade brain tumors tend to show strong contrast enhancement on post-contrast T1-w images suggesting that correction for T1 effects is important. The opposite has been observed in adult low grade gliomas6.Acknowledgements
Funded by: CRUK, EPSRC, MRC, NIHR, BCHRF, HHHOReferences
1Ho CY, et al. Neuroradiol. (2015) 57:299-306; 2Boxerman JL, et al. AJNR (2006) 27:859-67; 3Liu HL, et al. Med Phys (2011) 38:802-9; 4Ho CY, et al. AJNR (2016) 37:544-51; 5Dallery F, et al. Neuroradiol. (2017); 6Provenzale JM, et al. AJR (2002) 178:711-6.Figures
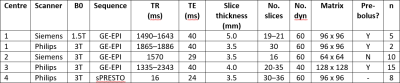
Table 1:
Summary of MR sequences
used at each center
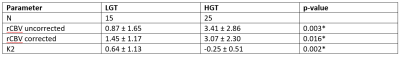
Table 2: Summary of
results for LGG and HGG groups
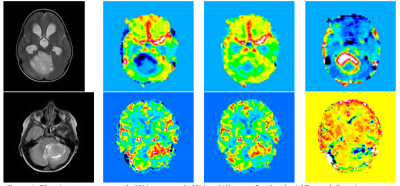
Figure
1: T2-w image, uncorrected rCBV, corrected rCBV and K2 maps for: (top) a LGT
case (pilocytic astrocytoma) which had rCBV = -1.09 ml/100ml prior to leakage
correction, rCBV = 2.17 ml/100ml after leakage correction and high positive K2
= 1.33 indicating T1-dominant effects, and (bottom) a HGT case (medulloblastoma)
which had rCBV = 3.22 ml/100ml before correction, rCBV = 2.63 ml/100ml and K2 =
-1.29 indicating T2-dominant effects
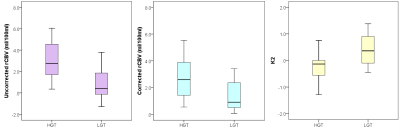
Figure
2: Box-and-whisker plots showing the range in values of uncorrected (left)
and corrected (center) rCBV and K2 (right) for the HGT and LGT groups