5417
Prevalence of brain lesions in a contemporary cohort of newborns with complex congenital heart disease prior to surgery1Centre for the Developing Brain, School of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2Paediatric Cardiology Department, Evelina Children's Hospital, London, United Kingdom
Synopsis
Infants born with congenital heart disease (CHD) are known to experience a distinct pattern of neurodevelopmental and behavioural impairment in later life. In this study, we scanned 64 infants with complex CHD prior to surgery, and used high-resolution structural, diffusion and susceptibility-weighted magnetic resonance imaging to understand the burden of brain lesions in a contemporary cohort. We characterised lesions and produced quantitative lesion maps, registered to a population template. We found a lower incidence of infarction than has been reported in previous comparable cohorts, and we explore the clinical reasons that may explain such variation.
Introduction
Congenital heart disease (CHD) is the most common congenital defect in newborns,1 affecting around 1% of births.2 Around one-half of children with complex CHD have some form of neurodevelopmental impairment.3 Studies of newborns prior to surgery have reported brain lesions including punctate white matter injury, periventricular leukomalacia and stroke with an incidence that varies between 19-52% of cases.4 We aimed to: 1) characterise brain lesions in infants with CHD prior to surgery and 2) assess which clinical factors are associated with brain injury.Methods
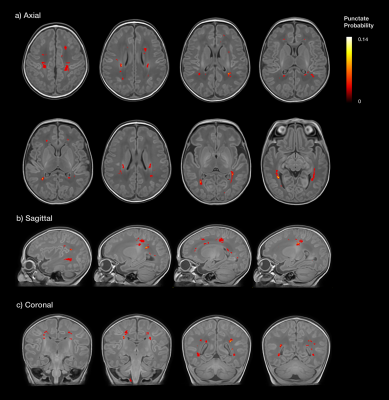
We recruited a prospective cohort of 64 newborns with CHD expected to require surgery within one year from the Neonatal Intensive Care Unit at St Thomas’ Hospital, London. MR imaging was performed in natural sleep on a Philips Achieva 3T system, including high-resolution T1-weighted MPRAGE (TR: 11ms, TE: 4.6ms, TI: 713ms, flip angle: 9º, voxel size: 0.8 × 0.8 × 0.8mm), T2-weighted multi-slice turbo spin-echo (TR: 12s; TE: 156ms, flip angle: 90º, slice thickness: 1.6mm acquired with an overlap of 0.8 mm; in-plane resolution: 0.8x0.8 mm), diffusion-weighted imaging (DWI; 300 diffusion directions, 4 phase-encode directions, b=0, 400, 1000 and 2600 s/mm2, voxel size 1.5x1.5x3mm) and fully flow-compensated 3D susceptibility-weighted imaging-spoiled gradient-recalled echo (SWI; TR 32ms; TE: 25ms; flip angle 12°; voxel size 0.4x0.4x1.8mm, SENSE factor 2). T1 and T2 images were reconstructed following the scan using a dedicated motion correction algorithm.5 Lesions were characterised by anatomical location and imaging appearance across each MR modality. Punctate lesions were segmented from T1-weighted images using ITK-SNAP (http://www.itksnap.org), and projected onto a population template generated using ANTS (http://stnava.github.io/ANTs). Statistical analysis was performed with SPSS V24 (IBM, New York).Results
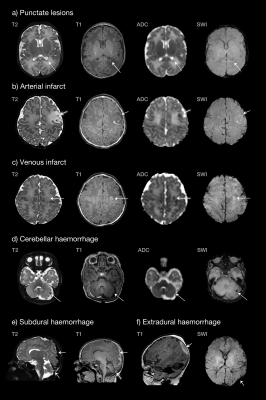
A complete dataset was acquired in 91% of infants. T2 and T1 were acquired in all subjects, DWI failed in 1 subject, and SWI failed in 5 subjects due to motion. The median gestational age (GA) at birth was 38.3 weeks (IQR 37.4-38.7), and 39.0 weeks at scan (IQR 38.4-39.6). Cardiac diagnoses included transposition of the great arteries (n=25), tetralogy of Fallot (n=12), coarctation of the aorta (n=10), hypoplastic left heart syndrome (n=4), pulmonary atresia (n=4), pulmonary stenosis (n=3), truncus arteriosus (n=3), tricuspid atresia (n=2), and large ventricular septal defect (n=1). Punctate lesions were identified in 36% (23/64), subdural haemorrhage in 31% (20/64), cerebellar haemorrhage in 9% (6/64), venous infarcts in 6% (4/64) and arterial infarction in 3% (2/64) (Figure 1). Twenty-three infants (36%) had no evidence of lesions on MRI and eight (13%) had more than one lesion type. Punctate lesions had no hypointensity on SWI in all cases, with either restricted (48%) or normal (52%) signal on mean diffusivity maps. Quantitative maps demonstrated punctate lesions distributed widely throughout the brain, particularly involving the frontal white matter, optic radiations and corona radiata (Figure 2). There was no significant relationship between brain injury and GA at birth, with no statistically significant difference between different cardiac diagnoses. Infants with TGA experienced the only arterial infarcts (2/25 TGA cases) and 75% of venous infarcts (3 of 4/64), which all followed balloon atrial septostomy. Subdural haemorrhage was positively associated with normal vaginal (p=0.02), ventouse delivery (p=0.01) and induction of labour (p=0.002). Induction was not associated with other injury types. Reduced cord arterial pH was associated with subdural haemorrhage (p=0.02), venous infarcts (p=0.03) and arterial infarction (p=0.04). Antenatal diagnosis of CHD had been made in 97% of cases (62/64), all of whom were born at our centre.Discussion
Punctate white matter injury commonly occurs in infants with CHD prior to surgery, at a rate over three times higher than healthy term infants,6 with a widespread distribution including frontal white matter, optic radiations, and corona radiata. While the prevalence of punctate white matter lesions is broadly consistent with previously reported CHD cohorts, we observed considerably fewer arterial infarcts. Indeed, previous studies have found arterial infarction rates up to three times higher in infants with TGA.7–9 In our study all infants with arterial infarction had undergone septostomy. Our data suggest that reduced cord arterial pH at birth may be a risk factor for brain injury. Punctate lesions were not associated with any specific clinical factor or CHD diagnosis. We suggest that the high rate of prenatal diagnosis of CHD and inborn delivery, which is probably associated with improved haemodynamic stability in the neonatal period,6 may be responsible for the low rate of major focal lesions observed in this study.Conclusion
We suggest that clinical factors including antenatal diagnosis of CHD and delivery at a cardiac centre may result in reduced lesion burden in infants prior to surgery. Future comparison of clinical practice between centres with different injury rates may reveal modifiable factors to improve neurodevelopmental outcomes in this population.Acknowledgements
This research was funded by the British Heart Foundation (FS/15/55/31649) and Medical Research Council UK (MR/L011530/1). This work received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/20072013)/ERC grant agreement no. 319456 (dHCP project), and was supported by the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z), MRC strategic grant MR/K006355/1 and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39: 1890–900.
2. Eurocat Prevalence Tables. http://www.eurocat-network.eu/accessprevalencedata/prevalencetables (accessed Nov 2, 2017).
3. Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental Outcomes in Children With Congenital Heart Disease: Evaluation and Management: A Scientific Statement From the American Heart Association. Circulation 2012; 126: 1143–72.
4. Mebius MJ, Kooi EMW, Bilardo CM, Bos AF. Brain Injury and Neurodevelopmental Outcome in Congenital Heart Disease: A Systematic Review. Pediatrics 2017; 140. DOI:10.1542/peds.2016-4055.
5. Cordero-Grande L, Hughes EJ, Hutter J, Price AN, Hajnal J V. Three-dimensional motion corrected sensitivity encoding reconstruction for multi-shot multi-slice MRI: Application to neonatal brain imaging. Magn Reson Med 2017; Early view. DOI:10.1002/mrm.26796.
6. Hughes E, Carney O, Tusor N, et al. The type and prevalence of incidental findings on magnetic resonance imaging of the low risk term born neonatal brain. In: ISMRM 25th Annual Meeting & Exhibition. 2017.
7. Peyvandi S, De Santiago V, Chakkarapani E, et al. Association of Prenatal Diagnosis of Critical Congenital Heart Disease With Postnatal Brain Development and the Risk of Brain Injury. JAMA Pediatr 2016; 170: e154450.
8. Dimitropoulos A, McQuillen P, Sethi V. Brain injury and development in newborns with critical congenital heart disease. Neurology 2013; 81: 241–8.
9. Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med 2007; 357: 1928–38.
Figures