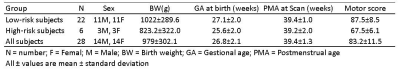
5416
Early Identification of Reduced Brain Functional Connectivity in Very Preterm Infants with Motor Impairments1Perinatal Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 2Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, United States, 3Pediatric Neuroimaging Research Consortium, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
Synopsis
Very preterm infants (<32 weeks gestational age) are at high risk for motor impairments. Investigation of brain network connectivity will improve our understanding of how brain organizational changes influence motor function and can result in improved individual risk stratification. In this work, we found reduction in functional connectivity in multiple motor and sensory regions, soon after birth, in very preterm infants at high risk of motor impairments assessed at 2 years of age. Our findings may enable mechanistic understanding and facilitate early, more accurate prediction of motor impairments.
Introduction
Very preterm infants (< 32 weeks gestational age) are at high risk for motor impairments, such as deficits in eye-hand coordination, sensorimotor integration, manual dexterity, and gross motor skills. 1-3 Studies report positive Early Intervention effects on motor outcomes, suggesting an urgent need for earlier identification, soon after birth, to take advantage of critical windows of brain development in early life. 4 Research supports the notion that severe motor impairments (i.e. cerebral palsy) may result from a perturbation of neural connection and communication. 5-7 Investigation of brain network connectivity will improve our understanding of how individual brain organizational changes influence motor function and can result in improved individual risk stratification.8-10 In this work, we hypothesize that very preterm infants who are later diagnosed with motor impairments at 2 years corrected age will present abnormal functional brain networks supporting motor functions, and we further hypothesize that this abnormality can be identified soon after birth.Methods
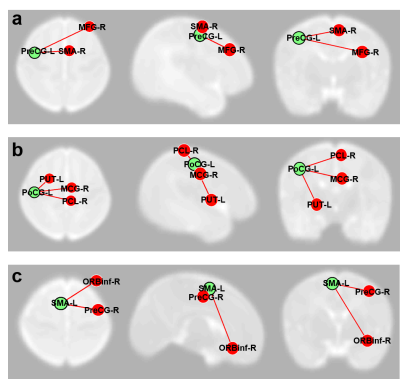
The study population was derived from a prospective cohort of very preterm infants from the NICU at Nationwide Children’s Hospital. Imaging specifications were: 3T GE HDx scanner with 8-channel infant head coil. T2w – TR/TE 11000/185 ms, FA 90°, resolution 0.35 x 0.35 x 2 mm3; functional connectivity MRI (fcMRI) – EPI TR/TE 3000/35 ms, FA 90°, resolution 2.8 x 2.8 x 3 mm3. Standardized Bayley-III testing was conducted at 2 years corrected age. The Bayley-III motor scores are on a scale of 50 to 150, with a mean of 100 and standard deviation of 15. We considered children with motor scores below 75 as high risk of developing motor impairments and dichotomized our subjects into 2 groups: high risk vs. low risk. The demographic information for these infants are provided in Table 1. Functional connectivity MRI (fcMRI) data were preprocessed using our established neonatal-optimized pipeline.11 In particular, we conducted non-brain tissue stripping, realignment, normalization to the University of North Carolina neonate brain template space12, and spatial smoothing. We segmented gray matter, white matter, and cerebrospinal fluid areas. Head motion artifacts were characterized using three-rotation and three translation parameters, plus six parameters representing their first-order temporal derivatives as well as scrubbing outliers detected by artifact detection & scrubbing tools (ART, MIT, Cambridge, US). To mitigate the impact of motion and physiological noise factors, we regressed out the confounding factors from the BOLD time series, including estimated subject motion parameters, and BOLD signal in white matter and cerebrospinal fluid areas. The resulting residual BOLD time series were band-pass filtered (0.008 Hz<f<0.08 Hz). We then analyzed resting state functional connectivity. Ninety region of interests (ROIs) were defined based on a neonatal automated anatomical labeling (AAL) atlas.12 The connectivity between each two ROIs were recorded as z-transformed Pearson’s correlation coefficient between their average BOLD times series. Last, we obtained ROI-to-ROI connectivity maps into a second-level general linear model to obtain population-level estimates. All of the above processing and analyzing steps were implemented in SPM8 (The MathWorks, Inc) and CONN functional connectivity toolbox (MIT, Cambridge, US).Results
The 6 infants that developed motor impairments exhibited marked reduction (at P<0.05 level) in functional connectivity in multiple motor and sensory regions (supplementary motor, pre- and post- central gyri) as compared to the 22 infants at low risk (Fig. 1).Conclusions
We compared functional connectivity between neonates at high vs. low risk of motor impairments assessed at 2 years of age via seed-based ROI-to-ROI analysis. In subjects at high risk, we found reduction in functional connectivity in multiple motor and sensory regions compared with subjects at low risk. These preliminary findings support the need for further investigations of brain functional connectivity as novel biomarkers for early identification/prediction of motor impairments.Acknowledgements
Funded in part by NIH grant R01 NS096037-01A1 from the National Institutes of Neurological Diseases and Stroke (NAP).
References
1. Serenius F, Kallen K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309:1810–1820.
2. Allen MC. Neurodevelopmental outcomes of preterm infants. Curr Opin Neurol. 2008;21(2):123–128
3. Hintz SR, Barnes PD, Bulas D, et al., Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics, 2015;135:e32–42.
4. Spittle A, Orton J, Anderson P, Boyd R, et al., Early developmental intervention programmes post-hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst Rev, 2012. 12: p. CD005495.
5. Hoon AH, Jr., Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Developmental medicine and child neurology. 2009;51(9):697-704.
6. Papadelis C, Ahtam B, Nazarova M, et al. Cortical somatosensory reorganization in children with spastic cerebral palsy: a multimodal neuroimaging study. Frontiers in human neuroscience. 2014;8:725.
7. Burton H, Dixit S, Litkowski P, et al., Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosensory & motor research. 2009, 26(4):90-104.
8. Kawahara J, Brown CJ, Miller SP et al., BrainNetCNN: Convolutional neural networks for brain networks; towards predicting neurodevelopment. Neuroimage, 2017, 146:1038-1049.
9. Kim DY, Park HK, Kim NS, et al., Neonatal diffusion tensor brain imaging predicts later motor outcome in preterm neonates with white matter abnormalities, Ital J Pediatr. 2016, 42(1):104.
10. Kwon SH, Vasung L, Ment LR, et al., The Role of Neuroimaging in Predicting Neurodevelopmental Outcomes of Preterm Neonates, Clinics in Perinatology, 2014, 41(1): 257-283.
11. He L, Parikh NA. Aberrant Executive and Frontoparietal Functional Connectivity in Very Preterm Infants with Diffuse White Matter Abnormalities. Pediatr Neurol, 2015; 53(4), 330-337.
12. Shi F., Yap PT, Wu G., et al., Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One, 2011. 6(4): p. e18746.
Figures