5415
Working memory training and brain structural and functional correlates in children born preterm1Murdoch Children's Research Institute, Melbourne, Australia, 2Department of Paediatrics, The University of Melbourne, Melbourne, Australia, 3Florey Institute of Neuroscience and Mental Health, Melbourne, Australia, 4Department of Medicine, Monash Medical Centre, Monash University, Melbourne, Australia, 5Monash Institute of Cognitive and Clinical Neurosciences, Monash University, Melbourne, Australia, 6King's College London, London, United Kingdom, 7Newborn Research, The Royal Women's Hospital, Melbourne, Australia, 8Department of Obstetrics and Gynaecology, The University of Melbourne, Melbourne, Australia
Synopsis
In a randomised controlled trial, we investigated if adaptive, computerised working memory training using Cogmed was associated with greater neural changes compared with a placebo training program. Participants were a population-based cohort of 91 school-age children born <28 weeks’ gestation or <1000 g birthweight. Children had structural, diffusion and task-based functional MRI before and two weeks following five weeks of Cogmed or placebo. There was little evidence for larger changes in cortical morphometry, white matter microstructure, or brain functional activity following Cogmed compared with placebo. In our study, Cogmed did not benefit brain structure or function in preterm-born children.
Introduction
There is some suggestion that computerised, adaptive working memory training using a program called Cogmed can improve working memory, which might translate into improved cognitive and academic outcomes in children born preterm.1-4 Cogmed may induce brain plasticity,5 however, few working memory training studies in children have examined brain changes,6-8 and none have used a randomised controlled design. This study aimed to investigate whether Cogmed is associated with structural, microstructural or functional brain changes in preterm children, compared with a placebo program. It was hypothesised that the Cogmed group would show larger changes in brain structure and function, particularly in brain regions involved in working memory, such as the frontal-parietal network,5 compared with the placebo group.Methods
Participants were derived from a geographical cohort of infants born extremely preterm (EP; <28 weeks’ gestation) and/or extremely low birthweight (ELBW; <1000 g) in Victoria in 2005. At 7 years of age, 91 EP/ELBW children were randomised to Cogmed or placebo, involving 45-minute training sessions, five days a week for five-seven weeks at home. In the Cogmed program, training difficulty changed based on the child’s current performance, whereas in the placebo program, training was set to a low difficultly level. Of the 91 children, 57 completed MRI pre-training and 2 weeks post-training. T1-weighted images were processed using FreeSurfer,9 with vertex-wise statistical analysis using the paired analysis stream. Diffusion-weighted images were processed using Diffusion Tensor Imaging, Neurite Orientation Dispersion and Density Imaging (NODDI),10 and the Spherical Mean Technique (SMT),11 and images were analysed using Tract-Based Spatial Statistics,12 with non-parametric permutation-based statistical analysis.13 Functional images, acquired while children performed the letter n-back task, were processed using the Functional MRI Expert Analysis Tool (FEAT). We performed initial descriptive analyses to examine whether brain cortical morphometry, white matter microstructure and functional activity changed from pre- to post-training in the Cogmed and placebo groups separately, and then performed interaction analyses between time point (pre- and post-training) and training group (Cogmed and placebo).Results
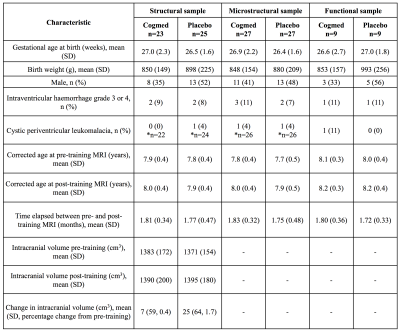
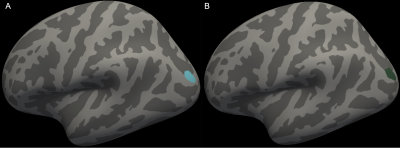
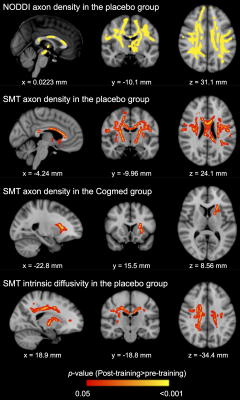
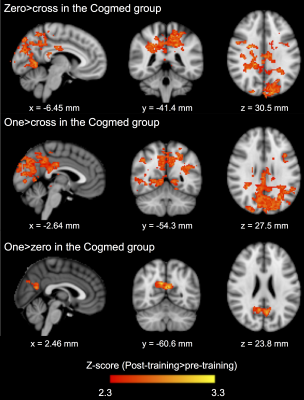
Baseline perinatal and demographic characteristics were similar in the Cogmed and placebo groups (Table 1). There was little evidence that cortical thickness or area changed from pre- to post-training, with the exception of one small cluster located in the left lateral occipital cortex, in which thickness decreased over time in the placebo group only (313 vertices, cluster size 216 mm2, 0.3% of total cortex; Figure 1A). There was also little evidence for a group-by-time interaction for cortical thickness and area, with the exception of a similar cluster in the left lateral occipital cortex, in which thickness decreased in the placebo group, but was relatively stable (or increased slightly) in the Cogmed group, from pre- to post-training (395 vertices, cluster size 277 mm2, 0.4% of total cortex; Figure 1B). There was evidence that axon density (from NODDI and SMT) and intrinsic diffusivity (from SMT) increased in many widespread tracts from pre- to post-training in the Cogmed and placebo groups (Figure 2). However, there was little evidence for group-by-time interactions for any diffusion measures. Functional activity during completion of the n-back task increased from pre- to post-training in the Cogmed group only, particularly in parietal/occipital brain regions (Figure 3). However, there was weak evidence for group-by-time interactions for functional activity.Discussion
Overall, there was little evidence for group-by-time interactions for cortical thickness and area, suggesting that cortical morphometry changed by similar amounts following training in the Cogmed and placebo groups. Thickness in part of the occipital cortex decreased more in the placebo group, although the meaning of this finding is unclear, and it should be interpreted with caution, particularly given the small cluster size. There was also little evidence that changes in white matter microstructure and functional activity following training differed between Cogmed and placebo groups. However, there were increases in functional activity in the Cogmed group only, in brain regions associated with working memory,14 which could indicate benefits of Cogmed on brain function. Previous studies also found changes in functional activity following Cogmed in children,6-8 although these studies did not include a placebo, so they could not determine if changes were specific to Cogmed training. Our lack of statistically significant differences in functional activity following training between Cogmed and placebo groups may suggest that changes in functional activity are not specific to Cogmed, or, they may reflect low statistical power from our small sample size, and so future studies with larger samples are needed.Conclusion
Cogmed compared with placebo did not result in training-induced changes to brain structure, microstructure or function in EP/ELBW children.Acknowledgements
No acknowledgement found.References
1. Grunewaldt KH, Lohaugen GC, Austeng D, et al. Working memory training improves cognitive function in VLBW preschoolers. Pediatrics. 2013;131(3):e747-754.
2. Grunewaldt KH, Skranes J, Brubakk AM, et al. Computerized working memory training has positive long-term effect in very low birthweight preschool children. Dev Med Child Neurol. 2016;58(2):195-201.
3. Lee CSC, Pei J, Andrew G, et al. Effects of working memory training on children born preterm. Appl Neuropsychol Child. 2017;6(4):281-296.
4. Lohaugen GC, Antonsen I, Haberg A, et al. Computerized working memory training improves function in adolescents born at extremely low birth weight. J Pediatr. 2011;158(4):555-561 e554.
5. Constantinidis C and Klingberg T. The neuroscience of working memory capacity and training. Nat Rev Neurosci. 2016;17(7):438-449.
6. Everts R, Murner-Lavanchy I, Schroth G, et al. Neural change following different memory training approaches in very preterm born children - A pilot study. Dev Neurorehabil. 2017;20(1):14-24.
7. Stevens MC, Gaynor A, Bessette KL, et al. A preliminary study of the effects of working memory training on brain function. Brain Imaging Behav. 2016;10(2):387-407.
8. Yoncheva YN, Hardy KK, Lurie DJ, et al. Computerized cognitive training for children with neurofibromatosis type 1: A pilot resting-state fMRI study. Psychiatry Res. 2017;266:53-58.
9. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781.
10. Zhang H, Schneider T, Wheeler-Kingshott CA, et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000-1016.
11. Kaden E, Kelm ND, Carson RP, et al. Multi-compartment microscopic diffusion imaging. Neuroimage. 2016;139:346-359.
12. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487-1505.
13. Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. Neuroimage. 2014;92:381-397.
14. Siffredi V, Barrouillet P, Spencer-Smith M, et al. Examining distinct working memory processes in children and adolescents using fMRI: Results and validation of a modified Brown-Peterson paradigm. PLoS One. 2017;12(7):e0179959.
Figures