5413
Feasibility of Quantitative Diffusion MR Tractography of the Vestibulocochlear Nerve in Children with Unilateral Profound Sensorineural Hearing Loss1Laboratory of Neuro Imaging, USC Stevens Neuroimaging and Informatics Institute, Keck School of Medicine of USC, Los Angeles, CA, United States, 2Division of Otolaryngology, Department of Surgery, UC San Diego School of Medicine, San Diego, CA, United States, 3Department of Otolaryngology, Keck School of Medicine of USC, Los Angeles, CA, United States
Synopsis
Diffusion MR tractography modeling of the vestibulocochlear nerve (VCN) has clinical value for characterizing hearing loss and surgical planning for cochlear implants; however, the neuroanatomy of the VCN presents technical challenges to image-based modeling. We conducted a feasibility study to evaluate VCN tractography in children with unilateral sensorineural hearing loss using advanced diffusion MRI acquisitions and modeling techniques. The results indicate tractography of the VCN is clinically feasible and quantitative tractography metrics reflect lateralization of hearing loss. We further found significant improvements from a reverse-phase encoding acquisition and multi-shell multi-compartment diffusion modeling.
Introduction
MR imaging of vestibulocochlear nerve (VCN) is important for the characterization of hearing loss and surgical planning of cochlear implants 3,14, and diffusion MR tractography is an emerging tool for VCN modeling 6,7,13. Previous studies have shown that tractography can be useful for modeling the VCN; however, the neuroanatomy of the VCN presents technical challenges to its visualization and quantitation with MRI, due to its small size and the presence of susceptibility induced artifact 1,2,5,8,9,10. We conducted a feasibility study of quantitative VCN tractography in children with unilateral severe to profound sensorineural hearing loss (SNHL) to evaluate advanced diffusion MR image acquisition and modeling techniques in this group. We specifically examined the relationship in laterality between tractography metrics and hearing loss, and evaluated multi-shell multi-compartment tractography compared to single-shell diffusion tensor tractography.
Methods
MR imaging data was collected from four children with SNHL (mean age = 10 y.o, three females, average hearing loss = 102.5 dB HL, average unaffected ear = 12.5 dB HL) on a Siemens Prisma 3T scanner. T2-weighted SPACE scans at 0.5 mm isotropic resolution were acquired along with a multi-shell HCP-style diffusion-weighted imaging (DWI) at 1.5mm isotropic resolution with 14 scans at b=0 s/mm2, 93 scans at b=1500 s/mm2, 94 scans at b=2500 s/mm2, and a complete repetition with reversed phase encoding in the anterior-posterior direction.
DWIs were denoised with a Rician non-local means filter and motion, susceptibility, and eddy current artifacts were corrected with FSL eddy4. Single diffusion tensor (SDT) models were fit using the b=1500 data, and the multi-compartment models (MCM) were fit using both shells using FSL bedpostx4. Deterministic streamline tractography reconstructions of each VCN were created with SDT and MCM models using the Quantitative Imaging Toolkit 12,15. 1000 seed points were sampled inside a 5mm radius sphere manually placed at the base of the pons in the brainstem. The study primarily focuses on the nerve, so tracking was stopped with manually placed box to exclude upstream connectivity. VCN tracks were selected by a 5mm radius sphere placed at the expected termination point of the VCN near the cochlea. Each tractography model was summarized by its track count, volume, average length, average density, and maximum density.
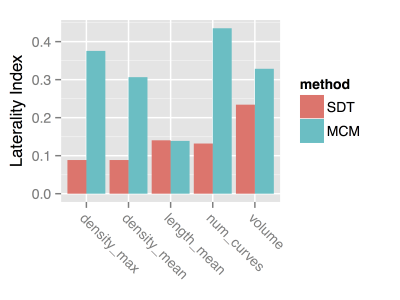
To compare imaging measures and SNHL, left and right metrics were combined to estimate a laterality index (LI) for each metric, which was defined by LI = 2.0 * (left – right) / (left + right). This is a dimensionless measure of laterality, where a positive value indicates greater values on the left, while a negative value indicates greater values on the right. The measure is scaled by the average value, so that the LI can be more directly compared across imaging metrics with different units, such as length, volume, etc.
Results
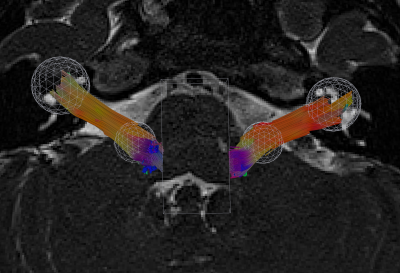
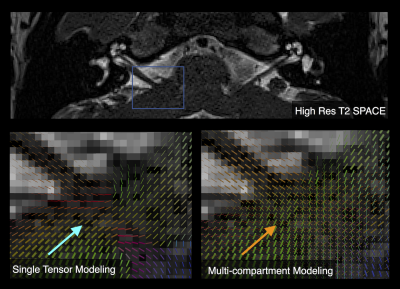
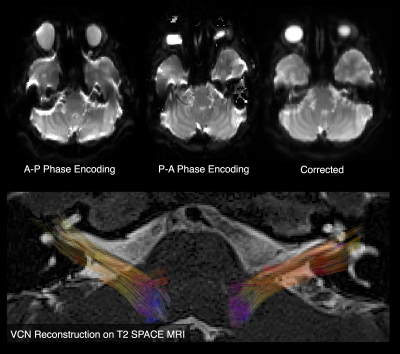
Tractography reconstructions of both left and right VCNs were found in all cases. A qualitative comparison showed shorter and smaller distribution of tracks consistent with the side with hearing loss (Fig 1). MCM revealed complex fiber architecture where the VCN enters the brainstem, while SDT modeling showed abrupt changes in fiber orientations (Fig 2). A quantitative analysis showed that the LI of track count, volume, length, and density reflected laterality consistent with subject’s side of hearing loss (Fig 3). SDT tractography had a mean LI of 0.137 and MCM tractography had a mean LI of 0.317, and a paired t-test showed that MCM LI was significantly higher than DT (p=0.028, t=-2.37, df=19). The improvements of MCM over SDT modeling were also present within each individual metric. We found that geometric distortion correction with FSL eddy was necessary to accurately co-register T2 and DWI datasets (Fig 4).
Conclusions
Quantitative tractography is clinically feasible in SNHL and provides a variety of promising quantitative imaging biomarkers with potential prognostic and diagnostic value. Multi-compartment modeling and correction of susceptibility induced artifact are recommended for improving accuracy and sensitivity in tractography-based analysis of the VCN. Compared to previous work, we demonstrate the strengths of tractography metrics obtained using advanced image acquisition and modeling techqiques, as they provide quantitative metrics and visualizations that are useful in characterizing SNHL. Several open problems remain, specifically to evaluate the subdivision of the VCN into cochlear and vestibular subcomponents and separation from the facial nerve. The results also demonstrated how multi-compartment diffusion modeling can reveal complex patterns of connectivity deeper into the brainstem; however, larger sample size is needed to investigate these in relation to SNHL.
Acknowledgements
This work was supported by NIH grant P41EB015922.References
1. Kabasawa, Hiroyuki, et al. "3T PROPELLER diffusion tensor fiber tractography: a feasibility study for cranial nerve fiber tracking." Radiation medicine 25.9 (2007): 462-466.
2. Hodaie, Mojgan, Jessica Quan, and David Qixiang Chen. "In vivo visualization of cranial nerve pathways in humans using diffusion-based tractography." Neurosurgery 66.4 (2010): 788-796.
3. Young, Nancy M., et al. "Pediatric cochlear implantation of children with eighth nerve deficiency." International journal of pediatric otorhinolaryngology 76.10 (2012): 1442-1448.
4. Jenkinson, Mark, et al. "Fsl." Neuroimage 62.2 (2012): 782-790.
5. Aggarwal, Manisha, et al. "Feasibility of creating a high-resolution 3D diffusion tensor imaging based atlas of the human brainstem: a case study at 11.7 T." Neuroimage 74 (2013): 117-127.
6. Rachakonda, Tara, et al. "Diffusion tensor imaging in children with unilateral hearing loss: a pilot study." Frontiers in systems neuroscience 8 (2014).
7. Huang, Lexing, et al. "Diffusion tensor imaging of the auditory neural pathway for clinical outcome of cochlear implantation in pediatric congenital sensorineural hearing loss patients." PloS one 10.10 (2015): e0140643
8. Yoshino, Masanori, et al. "Diffusion tensor tractography of normal facial and vestibulocochlear nerves." International journal of computer assisted radiology and surgery 10.4 (2015): 383-392.
9. Vos, Sjoerd B., et al. "Diffusion tensor imaging of the auditory nerve in patients with long-term single-sided deafness." Hearing research 323 (2015): 1-8.
10. Meola, Antonio, et al. "Human connectome-based tractographic atlas of the brainstem connections and surgical approaches." Neurosurgery 79.3 (2016): 437-455.
11. Yoshino, Masanori, et al. "Visualization of cranial nerves using high-definition fiber tractography." Neurosurgery 79.1 (2016): 146-165.
12. Cabeen, Ryan P., Mark E. Bastin, and David H. Laidlaw. "Kernel regression estimation of fiber orientation mixtures in diffusion MRI." Neuroimage 127 (2016): 158-172.
13. Kozin, Elliott D., and Daniel J. Lee. "Diffusion Tensor Imaging of the ‘Auditory Connectome’." The Hearing Journal 70.9 (2017): 8-9.
14. Fisher, Laurel M., et al. “Assessing the Benefit-Risk Profile for Pediatric Implantable Auditory Prostheses.” Therapeutic Innovation and Regulatory Science, September, 2017 (in press)
15. http://cabeen.io/qitwiki
Figures