5408
MRI-guided Focused Ultrasound to Improve Drug Delivery in Spinal Cord Injury1Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 2Department of Neurosurgery, University of Utah, Salt Lake City, UT, United States
Synopsis
Spinal cord injury (SCI) affects over 17,000 individuals each year in the United States, and most patients are left with some permanent paralysis. MRI-guided focused ultrasound (MRgFUS), when applied to the spinal cord with microbubbles to generate sonoporation, can transiently open the blood spinal cord barrier for effective drug delivery by breaking the glial scar. We used MRgFUS to increase permeability in the blood spinal cord barrier in a SCI rat model. Rats that underwent an MRgFUS procedure showed increased contrast caudal to the injury site, indicating drugs could potentially permeate these regions and assist with axonal regrowth.
Purpose
Spinal cord injury (SCI) affects over 17,000 individuals yearly in the United States,1 and most patients are left with some permanent paralysis. Limited treatment options only modestly improve outcomes.2 The glial scar that forms post-injury presents both a physical and a chemical barrier to axonal regrowth. This work investigates using MRI-guided focused ultrasound (MRgFUS) to increase permeability of the glial scar through opening the blood spinal cord barrier (BSCB). We investigated this hypothesis in a SCI rat model and evaluated BSCB opening with increased gadolinium contrast uptake.Materials and Methods
Sprague Dawley rats (N=46, 180-250g, female) were anesthetized and received laminectomies at T8-T10. A sham group received laminectomy only, while an injury group also received spinal cord compression with a 23g weighted clip at T9 for 1 minute (see Table 1 for study design). At week 4 post-surgery, injury rats were anesthetized, depilated over the target region, and positioned on an MRgFUS system (256-elements phased array, 940 kHz, IGT Inc., France) fitted with a custom rat holder in a 3T MR scanner (PrismaFIT, Siemens, Germany). 3D T1w high-resolution MR images (3D VIBE, FOV = 162x162x45 mm, Resolution = 0.2x0.2x0.4 mm, TR/TE = 6.21/2.94 ms, FA = 10°) were used to both position the rat and to assess the efficacy of the BSCB opening. MRgFUS sham rats received all treatments except sonication. Each treatment rat received 1-3 sonications (4 points, 2mm spacing, 20 ms bursts, 1 Hz pulse repetition frequency for 3 min, 1.0-2.1 MPa peak pressure). Microbubbles were injected intravenously before each sonication (200mL/kg, Optison, GE Healthcare, USA). BSCB opening was confirmed by an injection of a gadolinium contrast agent (0.25 mL/kg, Prohance, Bracco Diagnostic, USA, followed by 0.2 mL saline) and several contrast-enhanced T1w MR images were acquired (same parameters listed above). Pre-sonication the animals were imaged without and with a half dose of contrast to evaluate BSCB-opening due to the SCI. All animals underwent Basso, Beattie, and Bresnahan (BBB) locomotion assessment3 pre- and post-surgery and post MRgFUS treatment.Results
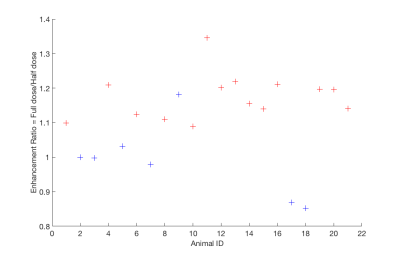
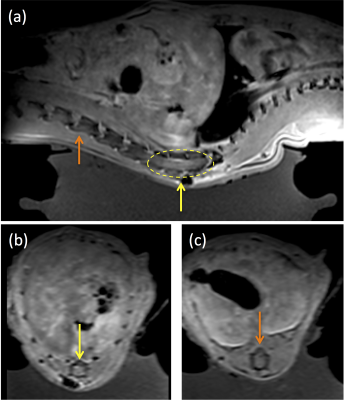
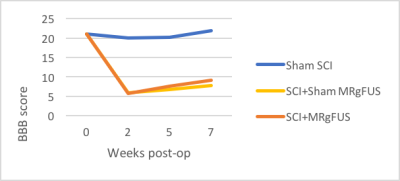
Increased contrast was seen in post-MRgFUS treated animals compared to MRgFUS sham animals. Figure 2 displays the enhancement ratio at the MRgFUS sonication site for treated (red) and sham (blue) animals. The enhancement ratio is defined as the ratio of a defined ROI in the spinal cord after a half-dose of contrast is given pre-MRgFUS sonication compared to after the administration of a full-dose of contrast given immediately post-MRgFUS. At the MRgFUS sonication site, group 2 treated animals had a significantly higher (P=0.0001) mean enhancement ratio of 1.17±0.067 (range: 1.09-1.35) compared to group 3 sham animals 0.99±0.11 (range: 0.85-1.18). In contrast, the enhancement ratio in a non-sonicated region of the spinal cord was not significantly different (P = 0.62) in the group 2 MRgFUS animals: 1.05±0.12 (range 0.78-1.29) when compared to group 3 sham animals 1.02±0.093 (range: 0.88-1.12). In Figure 3, CE-T1w MR images show (a) a sagittal view of spinal cord enhancement resulting from the MRgFUS BSCB opening (left side rostral and right side caudal to sonication region). MRgFUS sonications were applied at the injury site in the yellow dashed region. Axial views of (b) non-injured (orange arrow) and (c) injured and sonicated (yellow arrow) regions of the spinal cord are also shown. BBB scores were evaluated between SCI sham, MRgFUS sham, and MRgFUS treatment groups using ANOVA single factor analysis and Student’s t-test with a p value < 0.05. A significant difference was not seen between group 3 sham MRgFUS and group 2 treatment animals (p=0.11) on t-test. While a modest improvement was seen in BBB scores between group 3 sham MRgFUS and group 2 MRgFUS animals (Figure 4), the ANOVA showed a non-significant between group p value of 0.085.Summary and Conclusions
While other studies have demonstrated the ability to open the BSCB with MRgFUS,4 this is the first study to our knowledge that demonstrates MRgFUS can open the BSCB in a SCI rat model. Rats treated with MRgFUS showed increased contrast in spinal cord regions caudal to the injury site, indicating that drugs could potentially permeate these regions and assist with axonal regrowth. MRgFUS treatment alone indicated potential improvement in locomotion in SCI animals, and this will be explored in future studies, along with therapeutic medications that could have a significant impact on treatment options following SCI.Acknowledgements
No acknowledgement found.References
1. National Spinal Cord Injury Statistical Center, University of Alabama at Birmingham. National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. 2017. https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%20-%202017.pdf. Accessed 31 Oct 2017.
2. Cadotte DW, Fehlings MG. Spinal Cord Injury, A Systemic Review of Current Treatment Options. Clinical Orthopedics and Related Research 2011; 469: 732-741.
3. Basso DM, Beattie MS, Bresnahan JC. A Sensitve and Reliale Locomotor Rating Scale for Open Field Testing in Rats. Journal of Neurotrauma 1995; 12(1):1-21.
4. Weber-Adrian D, Thévenot E, et. al. Gene Delivery to the Spinal Cord Using MRI-guided Focused UItrasound. Gene Therapy 2015 July; 22(7):568-77.
Figures