5407
Oscillating Gradient Spin Echo (OGSE) Diffusion Tensor Imaging of the Human Spinal Cord: Application to Multiple Sclerosis1Philips Healthcare, Baltimore, MD, United States, 2Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
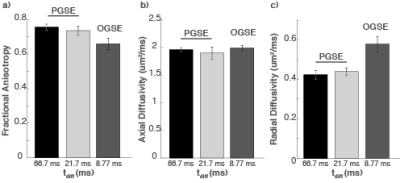
Compared to conventional pulsed gradient spin echo (PGSE) techniques, oscillating gradient spin echo (OGSE) provides access to shorter diffusion times, thereby enabling greater sensitivity to microstructure on smaller scales. Here, we report initial results of OGSE in the human spinal cord. Unlike in the brain, axial diffusivity in the spinal cord appears to be negligibly affected with diffusion time. Relative to the PGSE sequence (tdiff= 66 ms), the OGSE sequence (tdiff=8.77 ms) shows a 37% mean increase in radial diffusivity (RD) in healthy controls. When applied to MS, OGSE shows a larger difference in RD in comparison to healthy controls.
Introduction
The sensitivity of diffusion MRI measurements to microstructural changes on multiple tissue length scales depends on the diffusion time. Measuring diffusion MRI with a wider range of diffusion times may provide a more comprehensive characterization of tissues. Due to hardware constraints on clinical systems, conventional pulsed gradient spin echo (PGSE) diffusion techniques are inherently limited by the long diffusion times (usually > 25 ms), thereby restricting its sensitivity to restriction sizes of ~10 μm or more. Oscillating gradient spin echo (OGSE) provides the ability to achieve shorter diffusion times (e.g., 10 ms on clinical systems), and can enable greater sensitivity to diffusion on much smaller length scales (e.g., ~5 μm)1. However, OGSE typically requires long diffusion gradient durations, leading to long echo times as well as lower signal-to-noise ratios; consequently, OGSE imaging in humans has been mainly limited to the brain2-4. Here, we report the first results of OGSE in the human cervical spinal cord in vivo, with a clinical application to the spinal cord of multiple sclerosis (MS) patients.Methods
Acquisition: Two healthy volunteers (1M/1F, 27.5±7.78 years old) participated in this study, along with two relapsing-remitting MS patients (2F, 41.5±6.36 years old, mean Expanded Disability Status Score [EDSS]=3±0.71, mean duration of disease=6±2.83 years). Imaging was performed on a 3T whole body Philips scanner (Philips Ingenia, Best, Netherlands) using the two-channel body coil for excitation and a 52-channel dStream head/spine coil for reception. Diffusion sequences were acquired in the axial plane and consisted of a cardiac-triggered, reduced field-of-view (FOV), single-shot EPI with the following relevant parameters: FOV=68x49x10 mm3, resolution=1.25x1.25x10 mm3, SENSE (AP)=1.5, TE/TR=93 ms/3 beats (3000 ms), number of signal averages=5. Three diffusion sequences were acquired with 32 directions uniformly sampled on a half-sphere and mirrored with a b-value of 750 s/mm2, along with two non-diffusion-weighted images, with the following timing: (1) PGSE #1: Δ=70 ms, δ=10 ms, tdiff (effective diffusion time)=66.7 ms; (2) PGSE #2: Δ=25 ms, δ=10 ms, tdiff=21.7 ms; (3) OGSE: δ=35 ms, f=28.6 Hz, N=1, tdiff=8.77 ms. The acquisition time of each diffusion sequence was 9 minutes 47 seconds. An anatomical, multi-echo, gradient echo sequence was also acquired (TR/TE1/ ΔTE=752/7.1/8.8 ms) was used for registration and segmentation, along with identification of lesions for the MS patients.
Processing: Diffusion tensor calculation was processed using a nonlinear fit in Camino5, producing fractional anisotropy (FA), axial diffusivity (AD) and radial diffusivity (RD) maps for each acquired scheme.
Results
Healthy Controls: Figure 1 shows the averaged FA (Fig. 1a), AD (Fig. 1b), and RD (Fig. 1c), over both healthy controls from the three different diffusion sequences. With the two PGSE sequences (tdiff=66.7 and 21.7 ms), there are negligible differences in all three values (mean percent difference < 4.0%). However, when a short diffusion time (8.77 ms) is used with OGSE, there is a 37% increase in RD, but a negligible 1.53% increase in AD, in comparison to the longest diffusion time. Consequently, a mean 12.9% decrease in FA is observed when tdiff changes from 66.7 ms to 8.77 ms.
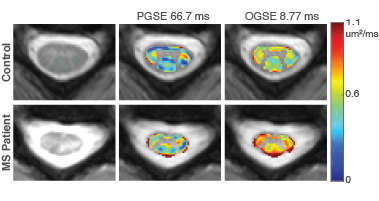
MS Patients: Figure 2 highlights the RD maps of the cervical spine for a representative control and MS patient. A larger increase in RD in the MS patient is observed with the OGSE sequence (mean percent change=46.5%), in comparison to the PGSE sequence (mean percent change=40.7%), which may indicate an increased sensitivity to pathological variations in MS using shorter diffusion times with OGSE.
Discussion
In this study, we demonstrate that high-resolution (1.25 mm in-plane) OGSE diffusion tensor imaging (DTI) can be achieved in human spinal cord in vivo with a diffusion time of 8.77 ms, one-third of the shortest diffusion time (~25 ms) achievable using PGSE. This increases the sensitivity to much smaller length scales in tissues and may, in turn, provide more comprehensive microstructural information. FA was found to decrease with decreased tdiff, which is consistent with previous reports of OGSE in human brain. However, the underlying dominant effects are very different: in the human brain, though RD changes with tdiff, a large tdiff dependency with AD is also observed3, suggesting DTI is sensitive to restrictions along the tensor principal direction, presumably caused by axon dispersion, undulation, beading, etc. Conversely, in the spinal cord, it appears that RD dominates the tdiff dependency, while AD shows negligible dependence. This may be due to the different sizes and arrangements of axons in the spinal cord compared with those in the brain. This suggests OGSE may provide novel insights in and improved modeling of nerve integrity in the spinal cord, with a different mechanism from previous reports of OGSE in the brain.Acknowledgements
The authors gratefully acknowledge funding from the following sources: National MS Society, Conrad Hilton Foundation, R21 (NIH/NINDS 1R21NS087465-01) and R01 (NIH/NEI R01 EY023240-05).References
1Gore, JC et al., NMR Biomed 2010; 23: 745-756.
2Van, AT et al., Magn Reson Med 2014; 71: 83-94.
3Baron, CA et al., Magn Reson Med 2014; 72: 726-736.
4Xu, J et al., NeuroImage 2014; 103: 10-19.
5Cook, PA et al., Proc ISMRM 2006, #2759.
Figures