5404
In vivo 31P magnetic resonance spectroscopy of human parotid gland1Department of Diagnostic Imaging and Nuclear Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan
Synopsis
This is the first in vivo study to investigate major phosphorus metabolites of human parotid gland with 31P magnetic resonance spectroscopy (MRS) using 3D chemical shift imaging (CSI) technique. Five healthy volunteers were measured on a clinical 3.0 T MRI scanner, and three of them were remeasured immediately after intake of tablets containing vitamin C. The spectra reveal large adenosine triphosphate (ATP) and phosphocreatine (PCr) intensities. Following intake of tablets containing vitamin C, decrease of β-ATP can be observed. In vivo 31P MRS can be used to assess bioenergetics of the human parotid gland within a reasonable scan time.
Introduction
The parotid gland is the largest of the salivary glands and secretes saliva via the parotid duct into the oral cavity to facilitate mastication and swallowing. Stimulated salivary production is largely (60–70% of total) derived from the parotid glands, with the balance from other glands1. Despite over 30 years of using 31P magnetic resonance spectroscopy (MRS) in research of human tissues (muscles, heart, liver, brain), to the best of our knowledge, no study of the human parotid gland has been reported in the English literature. The present work was designed to quantify the major metabolites of normal human parotid gland with 3D chemical shift imaging (CSI).Materials and Methods
Subjects and design: Five healthy volunteers (one female and four males; age 32-52y) were measured on a clinical 3.0 T MRI scanner, and three of them were remeasured immediately after intake of tablets containing about 200 mg of vitamin C.
MR experiment: Subjects were placed in a right lateral position in the magnet. T1 weighted ultrafast gradient echo images were collected with 10 mm slice thickness covering the right parotid gland. All 3D 31P spectroscopic imaging data were acquired on a 3.0 T MRI (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) using a 31P/1H loop transmit-and-receive RF coil and a 3D CSI-FID pulse sequence (TR/TE: 1000 ms/2.3 ms, bandwidth: 3000 Hz, 128 FID data points/zero-filling to 256). A 200 mm x 200 mm x 200 mm field of view (FOV) was phase encoded to 16 x 16 x 16 matrix resulting in a nominal voxel size of 2.2 cm x 2.2 cm x 2.5 cm or 12.3 ㎤. Weighted averaging (12 averages) was applied resulting in a total acquisition time of about 10 minutes.
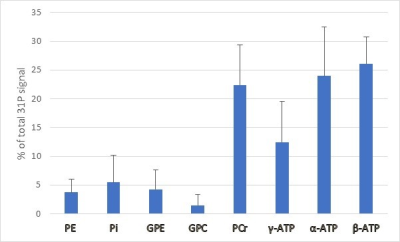
Data analysis: All spectra were analyzed with Syngo software (Syngo Spectroscopy Evaluation, Siemens Medical Systems, Erlangen, Germany). The following metabolites were fitted: phosphoethanolamine (PE), inorganic phosphate (Pi), glycerophosphoethanolamine (GPE), glycerophosphocholine (GPC), phosphocreatine (PCr), and adenosine triphosphate (ATP).
Results
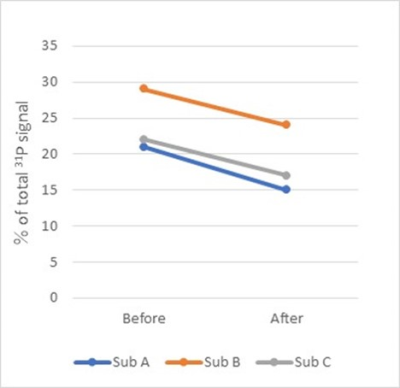
Figure 1 shows typical voxel position in the parotid gland. The voxel was set so that signals from other areas were not included. Good 31P spectra were obtained for all volunteer studies. The example of 31P MR spectrum is shown in Fig. 2. The spectrum reveals large ATP and PCr intensities, small PE, GPE, GPC, and Pi intensities. Percentage ratios of the metabolite spectral intensities to total phosphorus signal are shown in Fig. 3. The results represent mean spectral intensity ratio and standard deviations of five healthy volunteers. Following intake of tablets containing vitamin C, decrease of β-ATP can be observed. Compared to the first scans, β-ATP resonance over the total phosphorus signal was decreased from 24.0±4.6% to 18.7±3.9% (Fig. 4).
Discussion
This is the first in vivo study to investigate major phosphorus metabolites of human parotid gland with 3D CSI. Our results show that the proposed 31P MRS technique can measure the spectra of the parotid gland with acceptable quality and measurement time. The PCr signal in the parotid gland illustrates a high level of fast energy demand in the organ. This result in humans is consistent with data from previous animal models2. Furthermore, the results of sequential imaging with vitamin C intake show that it is possible to capture ATP changes due to salivary secretion. By applying this technique, 31P MRS can give us useful information on parotid gland function.Conclusion
In vivo 31P MRS can be used to assess bioenergetics of the human parotid gland within a reasonable scan time.Acknowledgements
None.References
- Dawes C, Wood CM. The contribution of oral minor mucous gland secretions to the volume of whole saliva in man. Arch Oral Biol 1973;18(3):337-342.
- Murakami M, Seo Y, Watari H. Dissociation of fluid secretion and energy supply in rat mandibular gland by high dose of ACh. Am J Physiol 1988;254(5 Pt 1):G781-787.
Figures