5400
Quantitative MR Neurography with Robust Fat Suppression1Philips Japan, Tokyo, Japan, 2Department of Clinical Radiology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, 3Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 4Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 5Philips Healthcare, Gainesville, FL, United States, 6Philips Healthcare Australia, North Ryde, Australia, 7Philips Healthcare Asia Pacific, Tokyo, Japan
Synopsis
MR neurography plays a major role in the diagnostic work-up of peripheral nerve pathologies and a quantitative evaluation based on T2 values can be clinically useful in estimating treatment effects and determining prognosis. Recently, we proposed a new sequence (SHINKEI-Quant) to add a quantitative information to MR neurography. To solve some issues caused by the current fat suppression techniques (SPAIR and STIR), we propose to combine SHINKEI-Quant with a new two-point dual-echo 3D DIXON-TSE (DE-mDIXON) technique. SHINKEI-Quant with DE-mDIXON simultaneously provides both MR neurography with high SNR, uniform fat suppression, and T2 maps with T2 values similar to conventional method. This sequence may be helpful to quantitatively assess nerve pathology.
Purpose
MR neurography plays a major role in the diagnostic work-up of peripheral nerve pathologies, substantially improving early diagnosis and patient management1-3. Furthermore, a quantitative evaluation by using T2 values can be clinically useful in estimating treatment effects and determining prognosis as well as diffusion tensor imaging4. Recently, we proposed a new sequence (SHINKEI-Quant5,6) to add quantitative information to MR neurography based on motion-sensitized driven-equilibrium (MSDE)-prepared fat-suppressed 3D T2-weighted turbo spin-echo (TSE)7,8. SHINKEI-Quant can simultaneously provide MR neurography and T2 maps within clinically feasible scan times5. This quantitative sequence can be combined with different types of fat suppression techniques. Spectral attenuated inversion recovery (SPAIR) or short tau inversion recovery (STIR) are frequently used in the brachial plexus. Although SPAIR facilitates excellent visibility of the nerves with high signal-to-noise ratio (SNR), image quality is often compromised by non-uniform fat suppression9. On the other hand, STIR provides uniform fat suppression, but suffers from reduced SNR of the nerves compared with SPAIR9. To solve these issues, we propose to combine SHINKEI-Quant with a new two-point dual-echo 3D DIXON-TSE (DE-mDIXON) technique10.Methods
Theory and pulse sequence:
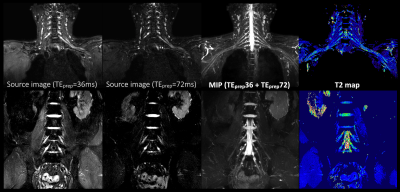
Conventional SHINKEI [Fig.1a] consists of fat-sat IR (SPAIR or STIR) and MSDE preparation, with a 3D T2-TSE sequence to acquire the data. MSDE is based on the improved MSDE (iMSDE11) with broadband offset independent trapezoid refocusing pulses9. The iMSDE module has a long duration to suppress flow and muscle signal simultaneously7. SHINKEI with DE-mDIXON [Fig.1b] consists of iMSDE and 3D DIXON-TSE, without fat-sat IR. Generally, in a two-point DIXON-TSE acquisition, the out-of-phase image is acquired by shifting the readout gradient with separate acquisition. To reduce the total imaging time, a bipolar multiecho acquisition was implemented to acquire both in-phase and out-of-phase echoes in the same TR10. SHINKEI-Quant applies two different iMSDE prep-times in the first half and the latter half of the acquisition. MR neurography is obtained by simple image addition. A T2 map is calculated on a pixel-by-pixel basis, by fitting magnitude image intensities from both iMSDE prep-times to a mono-exponential relaxation model. Accordingly, this sequence can provide both neurography images and T2 maps, without prolongation of the acquisition time [Fig.2].
Experiments:
A total of six volunteers were examined on 3.0T systems (Ingenia CX, Philips Healthcare). The study was approved by the local IRB, and written informed consent was obtained from all subjects.
To validate both the neurography images and the T2 maps obtained by SHINKEI-Quant with DE-mDiXON, coronal brachial plexus images were acquired and were quantitatively compared with conventional methods. Neurography images were compared with SHINKEI with SPAIR/STIR for image quality. We measured the apparent-SNR (aSNR, defined as the ratio of the signal mean to its standard deviation) of the nerves (average value of both the left and right dorsal root ganglion from C5 to C8) and sternocleidomastoid muscle, and the contrast ratio (CR) between the nerve and the muscle. Subsequently, the T2 maps were assessed for accuracy by comparing with a 2D multi-echo spin-echo (2D-SE) T2 mapping sequence. We measured the T2 value of the nerve and muscle respectively. The aSNR, CR and T2 values were assessed by using one-way repeated measures ANOVA and the post-hoc Tukey test. Imaging parameters common to all methods were: TR=2400ms, TE=63ms, echo spacing=5ms, ETL=100, voxel size=1.15*1.15*1.5mm3, iMSDE-Venc=1cm/s3, iMSDE-TEprep=36ms and 72ms, and scan duration=6min9s. In addition, SPAIR/STIR inversion delay was 250ms and DE-mDIXON delta TE was 1.05ms. The imaging parameters specific to 2D-SE were: TR=2400ms, TE=12ms*8, ETL=8, voxel size=1.15*1.15*3mm3 and scan duration=6min2s.
Results and Discussion
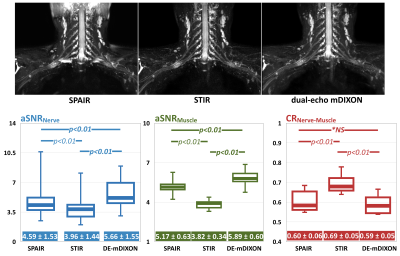
Fig.3 shows the comparison of representative images and aSNR/CR among SPAIR-SHINKEI, STIR-SHINKEI and DE-mDIXON-SHINKEI. The fat suppression using DE-mDIXON is better than using SPAIR. The aSNR of DE-mDIXON is significantly better than that of STIR and SPAIR. Although slightly lower than STIR, the CR of DE-mDIXON-SHINKEI showed similar values to SPAIR. DE-mDIXON-SHINKEI thus retains the fat suppression homogeneity of STIR-SHINKEI, while maintaining high SNR/CR of SPAIR-SHINKEI.
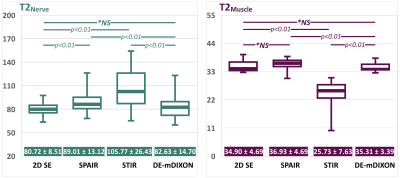
Fig.4 shows the comparison of T2 values between 2D-SE T2 mapping and SHINKEI-Quant with respective fat suppression techniques. The T2 values obtained with SHINKEI-Quant with DE-mDIXON in each tissue indicated values similar to 2D-SE, in contrast to the values from SPAIR/STIR due to the absence of an IR pulse with DE-mDIXON.
Fig.5 shows representative images of SHINKEI-Quant with DE-mDIXON images in the brachial- and lumber plexus. DE-mDIXON provides high SNR and contrast, uniform fat suppression, and accurate T2 values.
Conclusion
SHINKEI-Quant with DE-mDIXON simultaneously provides both MR neurography with high SNR and uniform fat suppression and T2 maps with T2 values similar to conventional 2D-SE. This sequence may be helpful to quantitatively assess nerve pathology.Acknowledgements
No acknowledgement found.References
1. Filler AG, et al. Application of Magnetic Resonance Neurography in the Evaluation of Patients with Peripheral Nerve Pathology. J Neurosurg. 1996;85:299-309.
2. Chhabra A, et al. High-Resolution 3T MR Neurography of the Brachial Plexus and Its Branches, with Emphasis on 3D Imaging. AJNR Am J Neuroradiol 2013;34:486–97
3. Vargas MI, et al. Three-Dimensional MR Imaging of the Brachial Plexus. Semin Musculoskelet Radiol. 2015;19:137-48.
4. Karampinos DC, et al. Diffusion Tensor Imaging and T2 Relaxometry of Bilateral Lumbar Nerve Roots: Feasibility of In-plane Iimaging. NMR Biomed. 2013;26:630–637.
5. Yoneyama M, et al. SHINKEI Quant: Simultaneous Acquisition of MR Neurography and T2 Mapping for Quantitative Evaluation of Chronic Inflammatory Demyelinating Polyneuropathy. Proc Intl Soc Mag Reson Med. 2016;24:1322.
6. Hiwatashi A, et al. Evaluation of Chronic Inflammatory Demyelinating Polyneuropathy: New Simultaneous T2 mapping and neurography method with 3D Nerve-Sheath Signal Increased with Inked Rest-Tissue Rapid Acquisition of Relaxation Enhancement Imaging (SHINKEI Quant). Proc Intl Soc Mag Reson Med. 2016;24:2261.
7. Yoneyama M, et al. Rapid high resolution MR neurography with a diffusion-weighted pre-pulse. Magn Reson Med Sci. 2013;12:111-9.
8. Kasper JM, et al. SHINKEI-a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging. Eur Radiol. 2015;25:1672-7.
9. Yoneyama M, et al. Motion-Sensitized Driven-Inversion (MSDI) for improvement of diffusion-prepared MR neurography (SHINKEI) in the brachial plexus. Proc Intl Soc Mag Reson Med. 2017;25:0854.
10. Wang X, et al. MR Neurography of Brachial Plexus at 3.0 T with Robust Fat and Blood Suppression. Radiology. 2017;283:538-546.
11. Wang J, et al. Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. J Magn Reson Imaging 2010;31:1256.
Figures
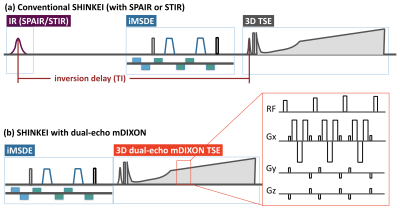
Fig.1. Methodological differences of conventional SHINKEI with IR-based fat suppression and dual-echo mDIXON.
Conventional SHINKEI (a) consists of fat-sat IR (SPAIR or STIR) and iMSDE preparation, with a 3D T2-weighted TSE sequence to acquire the data. The iMSDE module has a long duration to suppress flow and muscle signal simultaneously. SHINKEI with dual-echo mDIXON (b) consists of iMSDE and 3D DIXON-TSE only. To reduce the total imaging time, a bipolar multiecho acquisition was implemented to acquire both in-phase and out-of-phase echoes in the same TR.
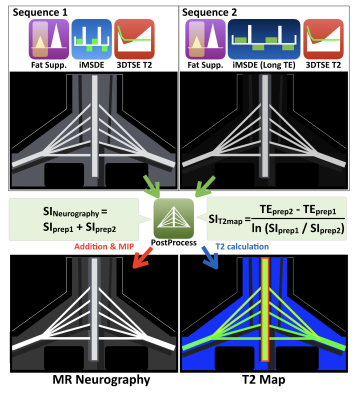
Fig.2. Theory of SHINKEI Quant sequence.
SHINKEI Quant applies two different iMSDE prep-times (36ms and 72ms) in the first half and the latter half of the acquisition. MR neurography is obtained by simple image addition and MIP post processing. A T2 map is calculated by a mono-exponential relaxation model.