5392
Improved brachial plexus visualization using an adiabatic iMSDE-prepared STIR 3D TSE1Department of Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar, Technische Universität München, München, Germany, 2Department of Diagnostic and Interventional Radiology, Klinikum rechts der Isar, Technische Universität München, München, Germany
Synopsis
The close proximity of blood vessels to the brachial plexus nerves can confound nerve visualization in the preferably used fat suppressed 3D T2 weighted sequences. Vessel suppression can be increased by means of an additional motion-sensitizing preparation (e.g. iMSDE). The aim of this work was the evaluation of STIR 3D-TSE in conjunction with an adiabatic T2 preparation incorporating iMSDE-based motion sensitization for MRN of the brachial plexus in a clinical routine-setting quantitatively and qualitatively. The additional motion-sensitizing iMDSE preparation reveals robust blood suppression, leading to higher CNR, increased conspicuity of the nerves, better image quality and less artifacts.
Purpose
Noninvasive magnetic resonance neurography (MRN) has become increasingly important in the diagnostic workout of brachial plexopathies and peripheral neuropathies1-4. Fat-suppressed 3D T2 weighted imaging, such as 3D TSE employing short tau inversion recovery (STIR) has been frequently used in MRN due to its good soft-tissue contrast and its high performance in high-resolution applications5, 6. A major concern in plexus MRN remains the immediate proximity of blood vessels to the plexus, which can confound the visualization of nerves. Vessel suppression in 3D MRN can be achieved by means of a motion-sensitizing preparation such as the improved motion-sensitized driven equilibrium (iMSDE) preparation, which has been shown to improve the visualization of nerve structures in various anatomies7-10. Another important consideration when employing a T2 preparation in MRN is the sensitivity to transmit B1 inhomogeneity effects. An adiabatic T2 preparation using a BIR-4 pulse has been shown to be robust to both B1 and B0 effects11-14. The purpose of this work was the evaluation of STIR 3D TSE in conjunction with an adiabatic T2 preparation incorporating iMSDE-based motion sensitization for MRN of the brachial plexus in a clinical routine-setting in comparison to STIR 3D TSE without a motion sensitizing prepulse.Methods
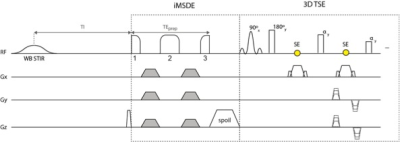
Subjects: 22 patients (8 female, 14 male; mean age: 45.5 ± 20.3 years) with different clinical implications participated in this study. MR Imaging: Brachial plexus was scanned on a 3T whole-body scanner (Ingenia, Philips Healthcare, Best, Netherlands) using a 16-channel torso coil, a 20-channel head-neck coil and the 12-channel embedded posterior coil. In addition to the standard MR protocol for the assessment of the brachial plexus (STIR 3D TSE; cor T1w DIXON TSE ± contrast agent; ax T2w TSE), STIR iMSDE 3D TSE was applied in all patients. A wide-band (WB) STIR pulse was used for robust fat suppression consisting of an adiabatic hyperbolic secant inversion pulse (length: 28.5 ms, total bandwidth: 1820 Hz). The iMSDE preparation used a modified BIR-4 pulse with gaps between the 3 RF components added to fit motion-sensitizing gradients (total duration BIR-4 RF pulse without gaps: 16 ms, frequency sweep: amplitude of 3800 Hz; see Fig. 1 for sequence diagram).
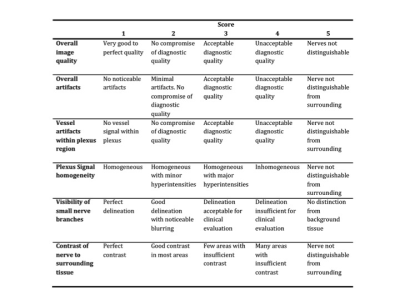
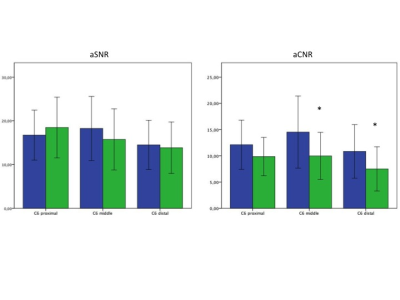
Analysis: Semiquantitative evaluation of the quality of maximum intensity projection (MIP) images was performed by two radiologists independently and blinded using 5-point grading scales. Fig. 2 shows the evaluated categories. Rating scores were compared between the two STIR-sequences using the nonparametric Wilcoxon signed-rank test. Apparent signal to noise ratios (aSNR) and apparent contrast to noise ratios (aCNR) were calculated form the original images by the following formulas: aSNR = Signal intensity (SI) nerve / standard deviation (SD) nerve; aCNR = SI nerve – SI adjacent soft tissue / SD nerve15. aSNR and aCNR were measured in tree different levels of the left C6 nerve (proximal, middle, distal). Paired student t-tests were used to assess the differences in aSNR and aCNR between both STIR-sequences on the same patient. p-values < 0.05 were taken as statistically significant.
Results
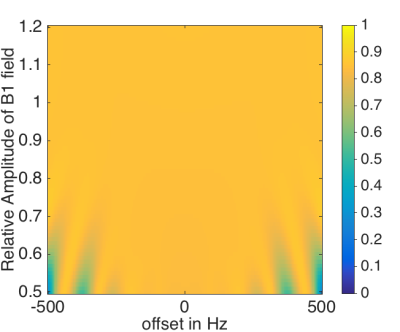
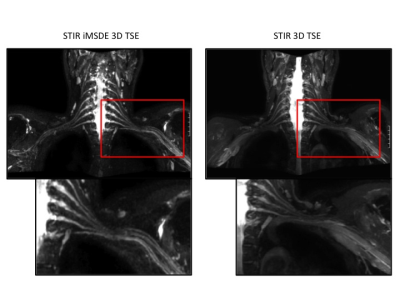
Signal simulations (Fig. 3) show the robustness of the used BIR-4 pulse in the presence of B1 inohomogeneity for imaging nerves for a wide range of B1 amplitudes. MIP images of both STIR 3D TSE sequences show a uniform homogeneous fat suppression; furthermore, the iMDSE prepulse reveals a good suppression of the arterial and venous vessel signal (Fig. 4). Pathologies of the brachial plexus were found in 8 patients. Compared to STIR 3D-TSE, STIR iMSDE 3D-TSE yielded significantly higher aCNRs in the middle and distal part (close proximity to vessels) of the left C6 nerve (14.5 ± 6.9 vs. 9. 9± 4.5, p=0.013*; 1.8 ±5.1 vs. 7.5 ± 4.2, p=0.038*) and a tendency for higher aCNR in the proximal part (12.1 ± 4.7 vs. 9.8 ± 3.6, p=0.084); no significant differences were found for aSNR between both sequences (Fig. 5). The semiquantitative ratings revealed a better overall image quality (p=0.014*), a higher contrast of nerve signal to surrounding tissue (p<0.001*), less overall artifacts (p=0.003*) and less artifacts by vessel signal (p<0.001*) when using the STIR iMSDE 3D-TSE compared to the STIR 3D-TSE.Discussion & Conclusion
The presented adiabatic iMSDE-prepared STIR 3D TSE method was shown to provide robust high-quality fat-suppressed T2-w imaging of brachial plexus nerves in the presence of B0 and B1 inhomogeneity. The incorporation of iMSDE-based motion sensitization was shown to provide robust blood suppression of vessels in close proximity to brachial plexus nerves, leading to higher CNR, increased conspicuity of the nerves, better image quality and less artifacts. Therefore, STIR iMSDE 3D TSE was shown to increase the diagnostic reliability of T2-w MRN of the brachial plexus and is recommended for clinical MRN examinations of this anatomy.Acknowledgements
The present work was supported by the European Research Council (grant agreement No 637164 – iBack) and Philips Healthcare.References
1. Du R, Auguste KI, Chin CT, Engstrom JW, Weinstein PR. Magnetic resonance neurography for the evaluation of peripheral nerve, brachial plexus, and nerve root disorders. J Neurosurg. 2010;112:362-371.
2. Martinoli C, Gandolfo N, Perez MM, et al. Brachial plexus and nerves about the shoulder. Semin Musculoskelet Radiol. 2010;14:523-546.
3. Tagliafico A, Succio G, Emanuele Neumaier C, et al. MR imaging of the brachial plexus: comparison between 1.5-T and 3-T MR imaging: preliminary experience. Skeletal Radiol. 2011;40:717-724.
4. Chhabra A, Lee PP, Bizzell C, Soldatos T. 3 Tesla MR neurography--technique, interpretation, and pitfalls. Skeletal Radiol. 2011;40:1249-1260.
5. Gerevini S, Agosta F, Riva N, et al. MR Imaging of Brachial Plexus and Limb-Girdle Muscles in Patients with Amyotrophic Lateral Sclerosis. Radiology. 2016;279:553-561.
6. Madhuranthakam AJ, Lenkinski RE. Technical advancements in MR neurography. Semin Musculoskelet Radiol. 2015;19:86-93.
7. Kasper JM, Wadhwa V, Scott KM, Rozen S, Xi Y, Chhabra A. SHINKEI--a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging. European radiology. 2015;25:1672-1677.
8. Yoneyama M, Takahara T, Kwee TC, Nakamura M, Tabuchi T. Rapid high resolution MR neurography with a diffusion-weighted pre-pulse. Magnetic resonance in medical sciences : MRMS : an official journal of Japan Society of Magnetic Resonance in Medicine. 2013;12:111-119.
9. Yoneyama M, Nakumara, M., Okukari, T., Tabuchi, T., Takemura, A., Obara, M., Ogura, J. High-resolution 3D volumetric nerve-sheath weighted RARE imaging (3D SHINKEI). Proceedings of the 19th Annual Meeting of ISMRM. 2011:2721.
10. Cervantes B, Kirschke JS, Klupp E, et al. Orthogonally combined motion- and diffusion-sensitized driven equilibrium (OC-MDSDE) preparation for vessel signal suppression in 3D turbo spin echo imaging of peripheral nerves in the extremities. Magn Reson Med. 2017.
11. Jenista ER, Rehwald WG, Chen EL, et al. Motion and flow insensitive adiabatic T2 -preparation module for cardiac MR imaging at 3 Tesla. Magn Reson Med. 2013;70:1360-1368.
12. Nezafat R, Ouwerkerk R, Derbyshire AJ, Stuber M, McVeigh ER. Spectrally selective B1-insensitive T2 magnetization preparation sequence. Magn Reson Med. 2009;61:1326-1335.
13. Weidlich D, Schlaeger S, Kooijman H, et al. T2 mapping with magnetization-prepared 3D TSE based on a modified BIR-4 T2 preparation. NMR Biomed. 2017.
14. Cervantes B. High-Resolution DWI of the Lumbar Plexus using B1-insensitive Velocity-Compensated Diffusion-Prepared 3D TSE. Proceedings of the 24th Annual Meeting of ISMRM. 2016.
15. Wang X, Harrison C, Mariappan YK, et al. MR Neurography of Brachial Plexus at 3.0 T with Robust Fat and Blood Suppression. Radiology. 2017;283:538-546.
Figures