5386
Alterations in white matter connectivity related to language comprehension in patients with Crohn’s disease in remission1Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Medicine, Division of Gastroenterology and Hepatology, Medical College of Wisconsin, Milwaukee, WI, United States, 3Department of Medicine, Division of Gastroenterology and Hepatology, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Reduced white matter integrity in patients with Crohn’s disease (CD) has been previously reported. However few studies have examined the behavioral implications of compromised WM integrity in these patients. Here we report results from an exploratory study investigating group differences in diffusivity measures in patients with Crohn’s disease in remission [compared to age and gender matched healthy control (HC) subjects] and examined their relationship to participants’ performance on a phonemic fluency task. CD patients demonstrate altered brain microstructural changes in regions associated with several cognitive functions including language processing.
Introduction
Previous neuroimaging studies have shown alternations in cortical thickness1 as well as functional brain activation patterns2 in multiple brain regions of Crohn’s disease (CD) patients in remission, which in certain regions are correlated with disease duration. A recent study examined the brains of IBD patients using DTI techniques and compared them with age-matched healthy controls3. That study found decreased white matter (WM) axial diffusivity in the right corticospinal tract and the right superior longitudinal fasciculus in IBD patients (with Crohn’s disease or Ulcerative Colitis) compared to controls, indicating possible alterations in WM integrity in these patients. But few studies have examined the relationship between compromised white matter integrity and deficits in cognitive processing in IBD patients. Here we used Diffusion Tensor Imaging (DTI) to examine the alterations in WM integrity in patients with CD in remission when compared to healthy controls and examined the relationship between DTI metrics and measures on the verbal (phonemic) fluency task.Methods
Data from 20 patients with Crohn’s disease in remission (12 male and 8 female, mean age = 35.85, SD = 15.78) and 20 healthy subjects (12 M and 8 female, mean age = 33.60, SD = 20.38) were analyzed in this study. All subjects participated in a phonemic verbal fluency (VF) task outside the scanner. High resolution 56 direction Diffusion Tensor Imaging (DTI) scan was obtained on a 3T GE scanner with the following parameters: TR/TE/θ = 9000 ms/60.60 ms/90°, FOV = 100 × 100 mm, slice thickness = 2 mm. A T1-wieighted anatomical scan was also obtained for co-registration. DTI metrics of WM coherence (fractional anisotropy-FA), axonal structure (mean, axial and radial diffusivity-MD, AD and RD) and a novel index of voxel diffusion (local diffusion homogeneity-LDH) were all computed using whole-brain tract-based spatial statistics (TBSS) with a MATLAB and FSL based toolbox named “Pipeline for Analyzing braiN Diffusion imAges (PANDA)” (http://www.nitrc.org/projects/panda/)4. The processing modules of FMRIB Software Library (FSL), Pipeline System for Octave and Matlab (PSOM), Diffusion Toolkit and MRIcron, are employed with PANDA. Briefly the methods include to convert DICOM to NIFTI, estimate the brain mask, cropping the raw images to cut off non-brain space, correction for eddy current effect, calculating the different diffusion metrics (fractional anisotropy- FA, mean, axial, radial diffusivity (MD, AD & RD) and local diffusion homogeneity (LDH) index) computing the mean of all the aligned FA images and creating a mean FA skeleton. The diffusion metric data from individual participants were then projected onto the skeleton. Finally, individual images with data on the skeleton were created and the resultant images used for voxel-wise statistical analysis on the skeleton. The fslmaths and tbss_skeleton commands of FSL were employed to extract diffusivity measures in 20 tracts under the JHU white matter atlas. Group differences in specific tracts were investigated using SPSS version 22.0 (p<.05).Results
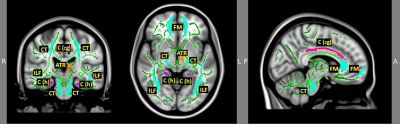
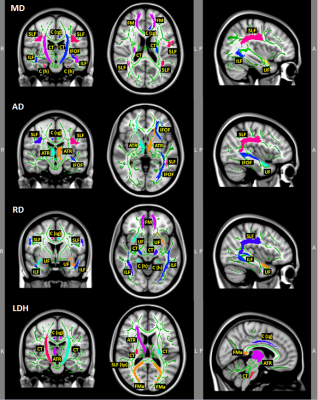
CD patients were not different in age (p = .71), handedness (p = .21), education (p = .39), or performance in verbal fluency score (p = .95) compared to the healthy controls. However, compared to the healthy controls, CD patients had significant decreased FA in regions with functions of motor (sensory), language, and interhemispheric sensory and auditory connectivity, all of which relate to language processing (Figure 1 and Table 1). Additionally, the two groups significantly differed in other regions with MD, AD, RD and LDH that also relate to language processing (Figure 2 and Table 1). Moreover, CD patients showed significantly increased FA, AD, LDH and lower RD with attention/pain processing function compared to healthy controls (see Figures 1-2 and Table 1).Discussion
These results suggest that although CD patients in remission score similarly to healthy controls in verbal fluency, they demonstrate altered brain microstructural changes in regions associated with several cognitive functions including language processing. It is posited that while currently unidentified CD-specific mechanisms lead to significant brain changes normally correlated with language processing, at least some CD patients can adapt their brain function to match verbal fluency outcomes of healthy controls.Conclusion
Despite confirmation of CD patients’ WM integrity alterations that normally associate with language processing changes, CD patients in remission did not score significantly differently in verbal fluency compared to age-, handedness-, and education-matched healthy controls. Future research to be conducted should investigate confounding variables of medication use and chronicity, as well as examine possible CD specific mechanisms related to WM integrity changes.Acknowledgements
This work was supported by the National Institute of Child Health and Human Development (grant number K12HD055894 to SS), and pilot funding from the UW-Madison Department of Radiology R&D (to SS) and the UW-Madison Department of Medicine (to SS), by the National Institute of Neurological Disorders and Stroke (grant number K23NS086852 to VP), American Heart Association (AHA) 2015 Innovation and AHA 2015 Midwest Affiliate Grant-in-Aid award (VP), by the National Institute of Health (grant numbers T32GM008692, UL1TR000427, T32EB011434). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors wish to thank our patients, and coordinators Jenny Vue and Jill Surfus for their help with patient recruitment and data collection, and the MR staff of the Wisconsin Institutes for Medical Research (WIMR) center.
Conflict of Interest Statement
Dr. SS is a consultant for UCB Biosciences, Inc. All the other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Nair V. A., Beniwal-Patel P, Mbah I, et al. Structural Imaging Changes and Behavioral Correlates in Patients with Crohn’s Disease in Remission. Front Hum Neurosci. 2016;10:460.
2. Beniwal-Patel P, Nair V. A., Mbah I, et al. Sa1886 altered brain functional activation and connectivity patterns in patients with crohn's disease in remission. Gastroenterology. 2016;150(4): S392.
3. Zikou AK, Kosmidou M, Astrakas LG, Tzarouchi LC, Tsianos E, Argyropoulou MI. Brain involvement in patients with inflammatory bowel disease: A voxel-based morphometry and diffusion tensor imaging study. Eur Radiol. 2014;24:2499–2506.
4. Cui Z, Zong S, Gong G. PANDA: A pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42.
Figures