5383
Spinal cord axonal diameter variations in the relapsing remitting EAE mouse model of multiple sclerosis1The Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia, 2College of Health Sciences, Taif University, Taif, Saudi Arabia, 3Centre for Integrated Preclinical Drug Development, Brisbane, Australia, 4School of pharmacy, The University of Queensland, Brisbane, Australia
Synopsis
This study aimed to determine the extent of axon diameter distribution changes in the lumbar spinal cord in C57BL/6 mice associated with experimental autoimmune encephalomyelitis (EAE). AxCaliber protocol was performed ex-vivo at 16.4T to analyze changes in mice with a range of EAE severity and the effect of treatment with alpha-lipoic acid (R-ALA), a nutraceutical compound that is recently known for suppressing microglial activation and CNS inflammation in EAE rodent models. AxCaliber showed axon diameter increases in mild relapsing remitting EAE group after R-ALA treatment. This might indicate a process of remyelination or other microstructural changes.
Introduction
Axon diameters have been shown to correlate with the transmission capacity of neurons in the central and peripheral nervous systems (CNS and PNS)[1]. The pathology of multiple sclerosis (MS) has been associated with demyelination and axonal loss[2]. Recently, MS-induced axonal injuries have also been shown to result in increased axonal diameter distributions (ADD) within the corpus callosum[3]. Changes in ADD is potentially a sensitive marker of disease severity reflected in dramatic presentations in MS patients, including loss of sensation, chronic pain and paralysis[4]. Previously, ADD was only accessible from histology of tissue biopsies. The AxCaliber protocol now allows non-invasive measurement of ADD using diffusion MRI[1].
This study aimed to use AxCaliber to measure ADD changes in the lumbar spinal cord (L-spine) of mild relapsing-remitting EAE (Mild-RR-EAE) mouse model of MS[5], and in a classical-EAE mouse model exhibiting chronic-progressive disease[2]. Additionally, we sought to determine whether ADD changes in mild-RR-EAE model could be alleviated using a chronic treatment of R-enantiomer of alpha-lipoic acid (R-ALA), which suppresses microglial activation and CNS inflammation in EAE[6].
Methods
Animals: The mild-RR-EAE was induced in groups of adult female C57BL/6 mice by subcutaneous (s.c.) immunization containing a mixture of 200μg of MOG35-55 and 45μg of Quillaja saponin (Quil-A) [5]. Two intraperitoneal (i.p.) injections (250ng) of pertussis toxin (PTX) were given on day 0 (first immunization day) and day 2. Sham (control) mice (n=5) received only adjuvants (Quil-A and PTX) as of the mild-RR-EAE group. Mild-RR-EAE mice were randomized to receive either vehicle (n=5) or R-ALA (n=5) treatment (10mg/kg/day) for ~21 days starting from day 15 post-immunization (p.i.) till day 36p.i. The classical-EAE model was induced by s.c. immunization with 75μg of MOG35-55 emulsified in 4mg (n=5) and 10mg (n=5) of Complete Friends Adjuvant (CFA), and two i.p. doses of 200ng of PTX (on day 0 and 2 respectively)[7].
Tissue preparation: All mice groups were euthanized at the chronic stage of the disease (day 28-35 p.i.), using an overdose of lethabarb and perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4 at 4°C, and post-fixed for ~24h. Samples were incubated with 0.2% Magnevist in PBS for 4 days at 4°C prior to MRI.
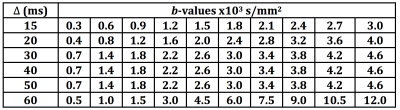
AxCaliber: Ex-vivo MRI was performed using a Bruker 16.4T vertical wide-bore microimaging system with a Micro2.5 gradient (1.5T/m) and 20mm volume coil (M2M Imaging, Brisbane, Australia). AxCaliber data were acquired using a stimulated echo DWI sequence with TE/TR=22/1500ms, 0.08x0.08x0.5mm3 resolution and diffusion encoding in the axial direction with δ=2.8ms and Δ=15-60ms. Variable Δ and b-values were as shown in Table 1. The acquisition time was 7.5h for each experiment.
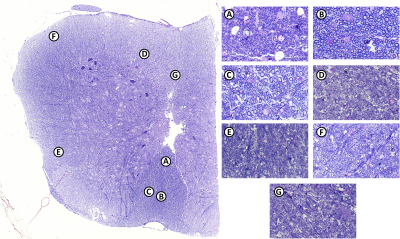
AxCaliber data was processed using a custom program in Matlab[1]. ROIs were drawn manually using ITKSnap (Figure 1).
Histology: Naive fixed L-spine (n=1) was embedded in epoxy and sectioned using a microtome at 0.5-micron slice thickness and stained using toluidine blue. High-resolution light microscope were obtained using 60x objective lens Nikon Eclipse Ti microscope. AxonSeg[8] was used to measure seven WM regions (similar to AxCaliber ROIs) at distinct locations (Figure 1).
Results
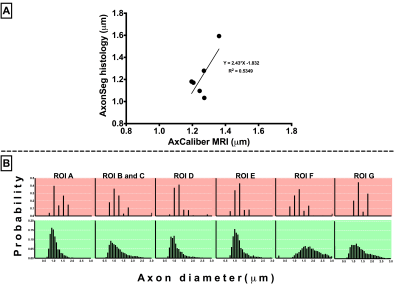
Preliminary correlation analysis using naïve data indicated moderate correlation of axon diameter measured by AxCaliber and AxonSeg (R2=0.53, p=0.09) (Figure 2-A). The profiles of ADD were similar for ex-vivo AxCaliber and AxonSeg from most WM ROIs (Figure 2-B).
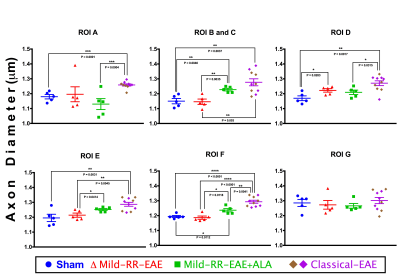
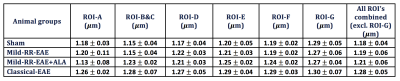
AxCaliber detected significant changes between all animal groups in the WM tracts, except in ROI-G (vestibulospinal tract) (Figure 3). Excluding ROI-G, the classical-EAE group showed a significant increase in the axon diameter compared to all groups in all ROIs (Table 2). R-ALA treatment in mild-RR-EAE group showed an increased axon diameter for ROIs B and C, E and F in comparison to the un-treated mild-RR-EAE, and also to sham groups. The sham and untreated mild-RR-EAE groups had similar axon diameters, except in ROI-D (rubrospinal tract) that was increased in the mild-RR-EAE compared to the sham group.
Discussion
AxCaliber observed increases in axon diameter in the mild-RR-EAE group after R-ALA treatment. The reason behind this is not yet clear, but may indicate a process of remyelination or other microstructural changes induced by R-ALA treatment. On the other hand, classical-EAE group revealed increases in axon diameter, which is consistent to that reported in a human MS study of the corpus callosum[3]. This may be an indication of increased intracellular space (cell-swelling), or an increase in the extracellular space in white matter due to demyelination.Conclusion
AxCaliber was successfully used to measure axon diameter changes in the EAE mouse spinal cord, which is promising as a clinical marker to monitor pathology in MS.Acknowledgements
We acknowledge support from the Queensland NMR Network and Australian National Imaging Facility for the operation of 16.4T MRI and the financial support of Taif University scholarship for Ahmad Joman Alghamdi.
References
1. Assaf, Y., et al., Axcaliber: A method for measuring axon diameter distribution from diffusion MRI. Magnetic Resonance in Medicine, 2008. 59(6): p. 1347-1354. 2. Khan, N. and M. Smith, Multiple sclerosis-induced neuropathic pain: pharmacological management and pathophysiological insights from rodent EAE models. Inflammopharmacology, 2014. 22(1): p. 1-22. 3. Huang, S.Y., et al., Characterization of Axonal Disease in Patients with Multiple Sclerosis Using High-Gradient-Diffusion MR Imaging. Radiology, 2016. 280(1): p. 244-251. 4. Duval, T., et al., g-Ratio weighted imaging of the human spinal cord in vivo. NeuroImage, 2017. 145(Part A): p. 11-23. 5. Khan, N., T.M. Woodruff, and M.T. Smith, Establishment and characterization of an optimized mouse model of multiple sclerosis-induced neuropathic pain using behavioral, pharmacologic, histologic and immunohistochemical methods. Pharmacology Biochemistry and Behavior, 2014. 126(0): p. 13-27. 6. Khan, N., et al., Antiallodynic effects of alpha lipoic acid in an optimized RR-EAE mouse model of MS-neuropathic pain are accompanied by attenuation of upregulated BDNF-TrkB-ERK signaling in the dorsal horn of the spinal cord. Pharmacology Research & Perspectives, 2015. 3(3): p. n/a-n/a. 7. Thell, K., et al., Oral activity of a nature-derived cyclic peptide for the treatment of multiple sclerosis. Proc Natl Acad Sci U S A, 2016. 113(15): p. 3960-5. 8. Zaimi, A., et al., AxonSeg: Open Source Software for Axon and Myelin Segmentation and Morphometric Analysis. Frontiers in Neuroinformatics, 2016. 10: p. 37.Figures