5381
Demyelination or not: increased radial diffusivity in Parkinson’s diseaseRuimeng Yang1, Pen Sun2, Xiangling Zeng1, Ajit George2, Ajit George2, xinhua Wei1, xinqing jiang1, and Sheng-Kwei Song2
1Guangzhou First People's Hospital, guangzhou, China, 2Washington University School of Medicine, St. Louis, Saint Louis, MO, United States
Synopsis
White matter (WM) integrity alteration has been noted in Parkinson’s disease (PD). Whether the increased radial diffusivity (RD) revealed in previous study using diffusion tensor imaging (DTI) in PD patients is suggestive of demyelinating etiology remains to be established. To further investigate white matter (WM) microstructure changes in PD, diffusion tensor imaging (DTI) and diffusion basis spectrum imaging (DBSI) were analyzed using Tract Based Spatial Statistics (TBSS). Both whole brain voxel-based group analysis and reginal analysis on the corpus callosum were performed. DBSI results suggest that axonal injury is present in PD while no myelin injury was seen.
Introduction
DTI has been widely used to describe WM integrity alternations in Parkinson’s disease (PD). Various studies reported increased radial diffusivity, known to reflect demyelination, in PD. Better imaging method is needed to separate and quantitatively assess individual pathological process in PD progression. DBSI was developed to quantitatively define and distinguish isotropic from anisotropic diffusion. [1,2] The multiple isotropic and anisotropic diffusion components derived by DBSI within WM represent infiltrating inflammatory cells, edema or tissue loss (non-restricted isotropic) and axon fibers (anisotropic). TBSS is one of the most popular whole-brain WM analysis tool employing skeleton-based registration process to greatly improve the accuracy and reliability through registration of individual subject to standard atlas. To further investigate brain WM microstructural changes in PD patients, both DTI and DBSI analyses on TBSS skeleton for cross group comparison were performed in current study to explore potential imaging biomarkers for PD. [3]Methods
Human Subjects: Procedures involving human subjects were all approved by the Institutional Review Board of Guangzhou First People’s Hospital. Every subject provided informed consent before their participation in the study. Sixteen PD patients and thirteen normal controls were included in this study. DBSI: All subjects underwent diffusion weighted MRI exams at 3.0T using a multi-b value diffusion weighting scheme (Magnetom Verio; Siemens, Erlangen, Germany). Diffusion-weighted images (DWIs) were collected with a 99 directions multi-b-value diffusion scheme using a single-shot spin-echo echo planar imaging sequence with the following key parameters: voxel size = 2.5×2.5×2.5 mm3; Maximum b-value = 2000 s/mm2; total acquisition time = 15 minutes. DTI and DBSI were computed using the in-house software developed using Matlab. Data analysis: The whole brain voxel-wise analysis of DTI and DBSI were carried out based on TBSS. DTI and DBSI indices were projected onto TBSS skeleton for statistical analyses. Nonparametric permutation tests were used for voxel-wise statistical analysis of the skeletons between health controls and PD patients. The significance threshold for group differences was set at p < 0.05. Thresholding was corrected for multiple comparisons across voxels by using the threshold-free cluster-enhancement option of the tool RANDOMIZE in FSL. Standard Regions of Interest (ROI) of the corpus callosum for each participant were selected based on the Johns Hopkins University (JHU) MNI template White Matter Parcellation Map (WMPM). Quantitative measurements of diffusion metrics were compared between groups and also correlated to clinical measures. Student t-test were used for reginal analysis at corpus callosum. The significance threshold for group differences was set at p < 0.05.Results and Discussions
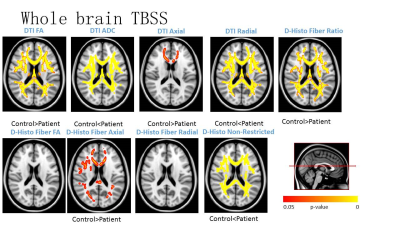
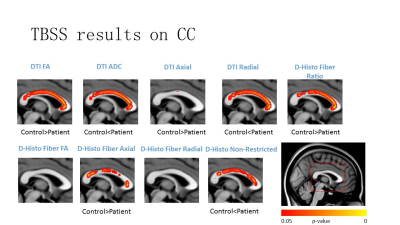
Voxel-based DTI and DBSI metrics revealed abnormal diffusion changes in widespread white matter regions in PD patients (Fig. 1). Same as previous reports, significantly increased DTI RD was observed in PD patients. DBSI RD suggest no demyelination at most of the WM regions. Instead, elevated DBSI non-restricted isotropic fraction suggested that the DTI RD increase was actually caused by the confounding effect of infiltrative inflammatory cells, edema or tissue loss. DBSI fiber fraction and AD suggest more severe axonal loss and axonal injury than DTI axial diffusivity (AD) detected. Regional analysis of DBSI on corpus callosum demonstrated the supportive results consistent with the above observations (Fig. 2).