5377
Diffusion Basis Spectrum Imaging and Diffusion Tensor Imaging in Mouse Model of Traumatic Brain Injury1Mallinckrodt Institute of Radiology, St. Louis, MO, United States, 2Department of Neurosurgery, SUNY Upstate Medical University, Syracuse, NY, United States
Synopsis
Traumatic brain injury (TBI) is a major cause of acquired brain injury in both children and adults. Traumatic axonal injury (TAI) is a major contributor to cognitive dysfunction followed by TBI. Diffusion Tensor Imaging (DTI) has shown to characterize TAI in non-invasive manner. However, DTI as a gross measure has limitations in its parameters in detection capability of underlying pathologies. Diffusion Basis Spectrum Imaging (DBSI) is an advanced imaging technique that has been applied to investigate nervous system pathology. Accurate characterization of brain pathologies in vivo, such as axonal injury, is extremely important in the study of changes that occur over time after TBI. To investigate these two methods in TBI data, we performed a comparison in mouse model between control and injured groups.
INTRODUCTION
Traumatic brain injury (TBI) is a major cause of acquired brain injury in both children and adults. Traumatic axonal injury (TAI) is a major contributor to cognitive dysfunction followed by TBI. Diffusion Tensor Imaging (DTI) has shown to characterize TAI in non-invasive manner [1]. However, DTI as a gross measure has limitations in its parameters in detection capability of underlying pathologies. Diffusion Basis Spectrum Imaging (DBSI) is an advanced imaging technique that has been applied to investigate nervous system pathology [2, 3, 4]. Accurate characterization of brain pathologies in vivo, such as axonal injury, is extremely important in the study of changes that occur over time after TBI. To investigate these two methods in TBI data, we performed a comparison in mouse model between control and injured groups.METHODS
Controlled Cortical Impact Model of TBI. After being anesthetized, mice were stabilized in a stereotaxic frame. An incision was made in the midline on the head, and a craniotomy in the right side of the head was performed with a dental burr drill. The cortical impact was initiated through the device graphical user interface of the impactor control software (Impact One, Leica). A 4-mm diameter flat face tip with a slightly rounded edge, a 1.5m/s strike velocity, a 2-mm strike depth from the surface of the dural, and 85 ms contact time were used for induction of cortical TBI. The procedure for sham operation was as the same as above except induction of TBI.
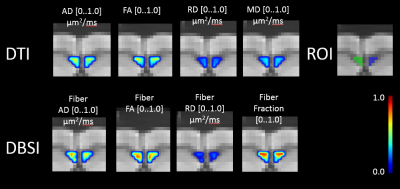
Nine mice in two groups were scanned with Agilent 4.7T scanner: 4 control, 5 post-trauma (see example in Figure 1). The images were acquired with a repetition period (TR) of 4 s, spin-echo time (TE) 33 ms, time between application of gradient pulses (delta) 18 ms, diffusion gradient duration (sigma) 5 ms, slice thickness 0.3 mm, field of view 2.0 cm, data matrix 128x128. Diffusion gradient applied in 99 directions with largest b-value 3000 mm/s2. The datasets were processed with a DBSI analysis package developed in-house with Matlab (MathWorks), including denoising.
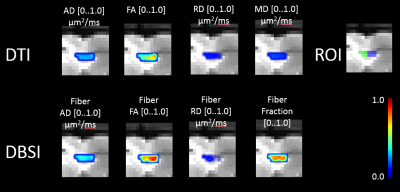
ROI tracing of corpus callosum region was delineated in the middle of corpus callosum (CC) region, ipsi and contralateral sides to the injury, drawing in coronal view slices, and consuting corresponding DTI FA maps in cases of uncertain regions in CC. See Figure 2 and Figure 3 for examples of delineation for control and post-trauma cases, correspondingly. The differences between the means of the parameter map intensity values were compared with Mann Whiney U test to see effect of injury to the neighboring tissue in corpus callosum region, and p-values were corrected for multiple comparisons with Bonferroni method. All statistical computations were implemented using R [5].
RESULTS
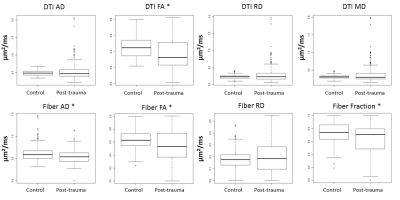
The comparison between control and post-trauma groups for DTI FA, Fiber FA, Fiber AD, Fiber Fraction had statistically significant difference (p<0.05), when sides of CC were pooled together. In post-trauma group, no difference was found between sides. General effect of injury was seen with DTI as slightly reduced axial diffusivity, reduced fractional anisotropy, and increased radial diffusivity, although only FA from DTI parameters was found to have statistically significant difference between groups. The DBSI based parameter values showed similar trends. In addition to FA of DBSI model anisotropic component, also reduced axial diffusivity and fiber fraction was found to be different after treatment, indicating axonal injury and fiber loss, which was not specificly readable from DTI parameters alone.CONCLUSION
We performed non-parametric comparison between parameter maps intensity values in control and post-trauma groups in TBI mouse model with 4 controls and 5 post-trauma cases. DTI based parameter maps showed differences in FA, indicating neural damage. However, the DBSI based parameters showed more prominent changes between the groups. Axonal Diffusivity and Fiber Fraction of DBSI showed potential in help of differentiating underlying pathologies in more specific manner than with conventional DTI technique alone.Acknowledgements
Harri Merisaari was supported by Sigrid Juselius Foundation, Finland.References
1. Mac Donald, C.L., et al., Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol, 2007. 205(1): p. 116-31.
2. Wang Y, Wang Q, Haldar JP, Yeh FC, Xie M, Sun P, Tu TW, Trinkaus K, Klein RS, Cross AH, Song SK. 2011b. Quantification of increased cellularity during inflammatory demyelination. Brain. 134: p. 3590–3601.
3. Song, S.K., et al., Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage, 2003. 20(3): p. 1714-22.
4. Song, S.K., et al., Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage, 2002. 17(3): p. 1429-36.
5. Team, R.C., R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. 2014.
Figures