5373
Comparison of diffusion MR models in lymph nodes at ultra high field1Champalimaud Research, Champalimaud Centre for the Unknown, Lisbon, Portugal, 2Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom
Synopsis
Diffusion MRI could play an important role for detection and characterisation of lymph nodes, however, the standard ADC metric lacks specificity and cannot accurately capture the signal decay. Multi-compartment models aim to represent the signal contribution of different water pools in order to better explain the acquired data. In this study, a rich dMRI dataset is acquired for ex-vivo lymph nodes and various diffusion models are fitted. The results show that including compartments which feature restricted diffusion improves the signal fit. Although such models have only a slight impact on lymph node differentiation, they could potentially provide more specific biomarkers.
Introduction
Diffusion MRI (dMRI) is a unique tool for non-invasive characterisation of tissue microstructure, which could play an important role for detection of lymph nodes metastases1,2, however, the apparent diffusion coefficient (ADC) is not always a reliable biomarker3. Moreover, ADC lacks specificity and cannot disentangle various effects which contribute to a change in signal4. Compartment models aim to represent the diffusion of different water pools inside the tissue, and have recently been applied for cancer imaging5,6,7.
Here, we use a rich dMRI protocol to investigate how different compartment models capture the measured signal in benign and malignant lymph nodes, and whether such techniques add value to their differentiation.
Methods
Institutional setting, approved by ethics committee: A total of 32 malignant nodes with different malignancy patterns (desmoplasia, tumour islands, solid infiltration, dispersed tumour cells) and 26 benign nodes (as defined by a dedicated pathologist) were included, originating from patients histopathologically staged as N+ after surgery with curative intent, without neoadjuvant therapy. The nodes were preserved in 4% formaldehyde and moved to a 1% PBS solution 24h before scanning.
Image acquisition: Nodal pairs were immersed in Flourinert® and imaged with a 16.4T Bruker® scanner using a Micro5 imaging probe. Imaging parameters: slice thickness: 0.7mm, in-plane resolution: 0.14x0.14mm, matrix size: 70x70, TR:2.8s, TE:6.5ms. Diffusion acquisition: stimulated echo acquisition mode (STEAM) diffusion sequences with gradient duration δ=1ms and diffusion times Δ={5,10,20,40,70,100,150}ms for b-values of 500 and 1000 s/mm2 and Δ={10,20,30,50}ms for b-values of 1500 and 2000 s/mm2 and 6 gradient directions for each parameter combination.
Data analysis: Acquired data was normalised for each diffusion time to account for T1 relaxation effects, then eight different diffusion models were fitted8: Ball, Tensor, Kurtosis, BallBall, BallSphere, ZeppelinBall, ZeppelinSphere, and BallSphereSphere. For the restricted diffusion, we use the expressions derived in 9.
The models were fitted to the signal averaged over whole node as well as over smaller ROIs (up to 10 per node, delineated by an experienced radiologist) and were compared based on the Bayesian Information Criterion (BIC) which accounts for the goodness of fit and penalizes increasing number of parameters. The ability of different models to differentiate between benign and malignant nodes was analysed using a general linear model (GLM) with binomial distribution, and the receiver operating characteristics (ROC) were computed
Results
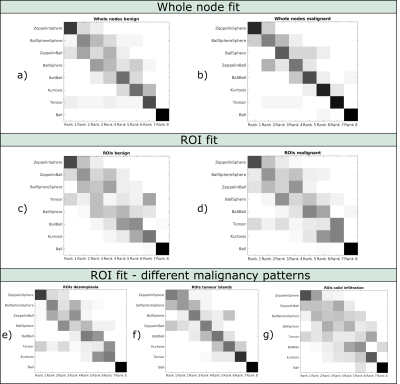
Figure 1 shows the model ranking for whole node fit (a and b) and ROI fit (c and d) for benign and malignant nodes as well as for ROI fit in different malignancy types e) – g). The ZeppelinSphere ranks first in most instances, while the simple Ball model (equivalent to ADC) consistently has the worst rank. In general, models with restriction are ranked better than models without restriction, nevertheless, the patterns are quite heterogeneous, especially when analysing individual ROIs.
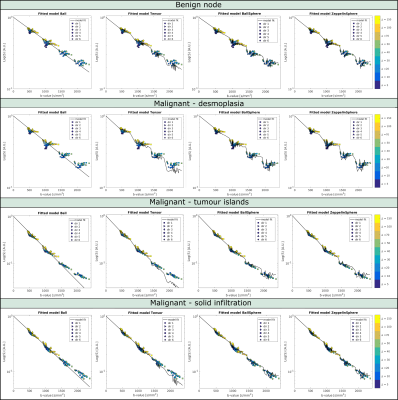
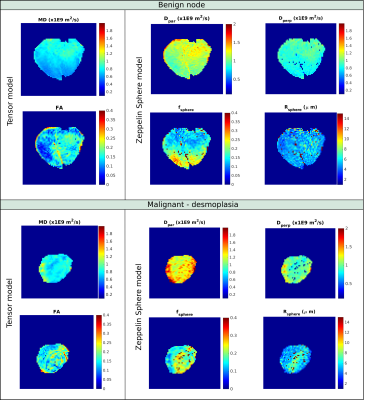
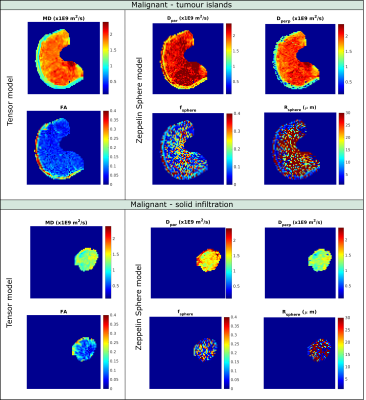
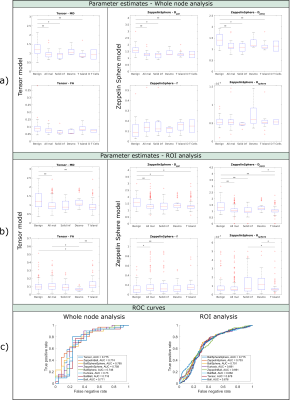
Figure 2 shows example data fits of four models (Ball, Tensor, BallSphere and ZeppelinSphere) for the whole node averaged signal in a benign as well as three different types of malignant nodes. A more pronounced time dependence is seen in benign and desmoplasia nodes compared to nodes with tumour islands and solid infiltration. Parameter maps of voxel wise fit for the Tensor and ZeppelinSphere models are shown in Figures 3 and 4. Figure 5a) and b) shows boxplots of parameter estimates for the Tensor and ZeppelinSphere models. The tensor mean diffusivity, as well as the diffusivities from the Zeppelin compartment show significant differences between benign and malignant, with the difference mostly driven by nodes with tumour islands and solid infiltration.The ROC curves in Figure 5c) show that for whole nodes, the Tensor and ZeppelinBall models best differentiate between benign and malignant nodes, while for ROIs, the models with restriction yield a better result. Moreover, carefully choosing which model parameters to include in the ROC analysis might further improve the results.
Discussion
This study uses a rich dMRI data set to investigate which compartment models best describe the signal in lymph nodes. Models accounting for restricted diffusion seem to fit the data better, which can provide insight into the diffusion mode in the nodes. In lieu of histology, we take care not to assign specific compartments to biological counterparts. For lymph node differentiation, compartment models only slightly improve the results, and future studies will strive to make a better distinction between malignant and benign nodes.Conclusion
This study shows that accounting for restricted diffusion improves the data fit of dMRI signal in ex-vivo lymph nodes. Although this has only a slight impact on lymph node differentiation, it might provide more specificity towards tissue microstructure.Acknowledgements
This study was supported by funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Starting Grant, agreement No. 679058), EPSRC (grant number M507970) and Champalimaud Foundation.References
1. Ogawa, M., et al., The Usefulness of Diffusion MRI in Detection of Lymph Node Metastases of Colorectal Cancer. Anticancer Research, 2016. 36(2): p. 815-9.
2. Yasui, O., M. Sato, and A. Kamada, Diffusion-weighted imaging in the detection of lymph node metastasis in colorectal cancer. Tohoku J Exp Med, 2009. 218(3): p. 177-83.
3. Heijnen, L.A., et al., Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. Eur Radiol, 2013. 23(12): p. 3354-60.
4. Liu, C., R. Bammer, and M.E. Moseley, Limitations of apparent diffusion coefficient-based models in characterizing non-gaussian diffusion. Magn Reson Med, 2005. 54: p. 419-28.
5. Panagiotaki, E. et al., Non-invasive quantification of solid tumour microstructure using VERDICT MRI. Cancer Research, 2014. 74: p. 1902-1912.
6. Liang, S., et al., Information-based ranking of 10 compartment models of diffusion-weighted signal attenuation in fixed prostate tissue. NMR Biomed, 2016. 29: p. 660-71.
7. White, N.S., et al., Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res, 2014. 74: p. 4638-52.
8. Panagiotaki, E., et al., Compartment models of the diffusion MR signal in brain white matter: a taxonomy and comparison. Neuroimage, 2012. 59(3): p. 2241-54.
9. Alexander, D.C. and T.B. Dyrby, Diffusion imaging with stimulated echoes: signal models and experiment design. arXiv, 2013: p. 1305.7367.
Figures