5367
Impact of time-of-day on diffusivity measures of brain tissue derived from diffusion tensor imaging1National Institute of Mental Health, Bethesda, MD, United States, 2National Institute of Biomedical Imaging and Bioengineering, Bethesda, MD, United States, 3National Institute of Neurological Disorders and Stroke, Bethesda, MD, United States
Synopsis
Although diurnal fluctuations in DTI-based measures of brain tissue properties have been reported, the underlying mechanism has not been investigated. Here, we processed multi-shell diffusion data using the conventional monoexponential tensor model as well as with a dual-compartment tensor model and assessed whether, time-of-day (TOD) fluctuations in DTI measures are due to changes in the magnitude of a CSF-like water compartment, or due to changes in the diffusion properties of parenchymal water. Our results suggest that TOD effects are due to fluctuations in the magnitude of a CSF-like water compartment rather than changes in the diffusion properties of parenchymal water.
Purpose
To assess the impact of time-of-day (TOD) on DTI-based measures and explore the underlying mechanism.Introduction
There is growing recognition that diurnal changes in brain physiology impact MRI measures of brain structure and function1,2,3. These fluctuations may have a physiological origin, since they have been detected using different MRI modalities, and cannot be explained by factors that are typically known to confound MRI measures. A prime candidate for further investigation is cerebrospinal fluid (CSF), or CSF-like water, since our recent findings based on analysis of T1W images suggested that the TOD-related decrease in the apparent parenchymal volume, was correlated with an increase in apparent CSF volume2. If CSF volume was indeed increasing from AM to PM, then the Trace of the diffusion tensor (i.e. mean diffusivity x 3) would be expected to increase from AM to PM as well. However, with the single compartment DTI model, it would be impossible to assess if the increase in Trace is due to an increase in the magnitude of a CSF-like/freewater water compartment or due to an increase in water diffusivity within the parenchyma. Here, we used a dual-compartment tensor model4 that allowed us to address whether: a TOD-related increase observed in Trace can be caused by an increase in the volume fraction of the CSF-like water pool or by an increase in the diffusivity of the parenchymal water pool.Methods
A group of 19 healthy, young adults were recruited as part of a larger study and were scanned during multiple visits on a 3 Tesla GE MR750 scanner using a GE 32-channel head coil. Each visit included scan sessions at 10 AM and 2 PM, with 2 hours of rest in between scans. Whole-brain single-shot echo-planar (EPI) diffusion weighted images (DWI) were acquired with the following parameters: TE/TR = 86.9/20996 msec, b-values (volumes) (b=0(10), b=300(10), and b=1100(60) sec/mm2), voxel size = 2mm isotropic, and acceleration factor (ASSET) = 2. Each individual’s DWIs were corrected for distortions and artifacts using TORTOISE5. The diffusion tensor (DT) was computed using the monoexponential tensor model as well as a biexponential dual-compartment model4 with one compartment modeled as a tensor with diffusivity constrained to be < 3×10–3 mm2/s and the other modeled as an isotropic compartment with fixed diffusivity (the diffusivity of free water at 37 °C: = 3×10–3 mm2/s). Subject-specific templates of each subject’s DT were created and these were used to create a study-specific template using DR-TAMAS6. Finally, the displacement fields from native to subject-specific template and to the study-specific template were applied to the DT’s in native space to bring all the DTI-maps to the study space. To test whether DTI measures including Trace, axial diffusivity (AD), radial diffusivity (RD), and fractional anisotropy (FA) fluctuate from AM to PM, we used permutation-based nonparametric statistical analysis using the threshold-free cluster enhancement method7 and corrected for multiple comparisons with family-wise error (FWE) correction (p < .05).Results
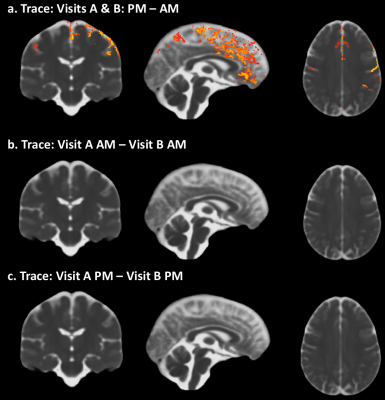
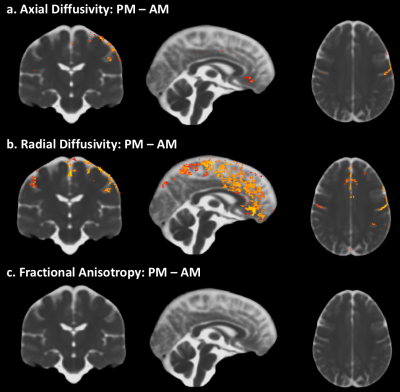
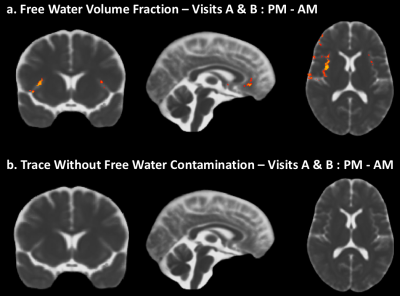
A significant increase in Trace from AM to PM can be observed along the midline and specific brain regions (fig1a). In contrast, such differences are absent when comparing Trace between the two AM visits and PM visits, a week apart (fig1b, c). Measures of diffusivity such as AD (fig2a) and RD (fig2b) also show an increase from AM to PM, predominantly in the same brain regions that show an increase in Trace, while FA (2c) was not impacted. The dual compartment analysis suggests an increase in CSF-like volume fraction from AM to PM in regions that overlap with the increase in Trace (fig3a). Finally, removal of the contribution from CSF-like free water from Trace eliminates the TOD-related increases in Trace observed with the monoexponential fitting.Discussion
Our study reveals a clear increase in Trace, RD and AD from morning to afternoon. That such changes in DTI measures can be detected even in the absence of an experimental manipulation suggests that controlling for the effect of TOD is crucial for proper interpretation of apparent differences in DTI measures of brain tissue properties.The increase in Trace appears to be predominantly in the grey matter – CSF boundaries, and could be attributed to different pools of fast-diffusing water (e.g. water in perivascular spaces, CSF) or pseudo diffusion effects due to intra-voxel incoherent motion8. The dual compartment analysis suggests that the TOD-related increase observed in Trace may be caused by an increase in the volume fraction of the CSF-like water pool and not due to any changes in the diffusivity of the parenchymal water pool.Acknowledgements
This work was supported by Intramural Research Programs of NIBIB and NIMH. Salary support for CT was provided by funding from the Department of Defense in the Center for Neuroscience and Regenerative Medicine.References
1. Hodkinson, D. J. et al. Circadian and homeostatic modulation of functional connectivity and regional cerebral blood flow in humans under normal entrained conditions. Journal of Cerebral Blood Flow & Metabolism 34, 1493-1499 (2014).
2. Trefler, A. et al., Impact of time-of-day on brain morphometric measures derived from T 1-weighted magnetic resonance imaging. NeuroImage 133, 41-52 (2016).
3. Jiang, C. et al., Diurnal microstructural variations in healthy adult brain revealed by diffusion tensor imaging. PloS one 9, e84822 (2014).
4. Pierpaoli, C., & Jones, D., Removing CSF Contamination in Brain DT-MRIs by Using a Two-Compartment Tensor Model. Proceedings of the International Society for Magnetic Resonance in Medicine. Presented at the ISMRM, Kyoto, p. 1215. (2004)
5. Pierpaoli, C. et al., TORTOISE: an integrated software package for processing of diffusion MRI data. Proceedings of the International Society for Magnetic Resonance in Medicine. Presented at the ISMRM, Stockholm, p. 1597 (2010).
6. Irfanoglu, M. O. et al., DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures. NeuroImage 132, 439-454 (2016).
7. Smith, S.M. & Nichols, T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localization in cluster inference. NeuroImage, 44(1), 83e98. (2009)
8. Le Bihan, D. et al., MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161, 401-407 (1986).
Figures