5354
EDDY QC: Automated quality control for diffusion MRI1Wellcome Centre for Integrative Neuroscience (WIN) - FMRIB, University of Oxford, Oxford, United Kingdom, 2Department of Psychiatry, University of Oxford, Oxford, United Kingdom, 3Sir Peter Mansfield Centre, School of Medicine, University of Nottingham, Nottingham, United Kingdom
Synopsis
Given the very large number of individual datasets acquired in recent population imaging studies, it is becoming essential to automate data quality control (QC). Here we present an automated QC framework to assess diffusion MRI data both at the single subject and group levels. The QC metrics are derived through different stages of FSL’s pre-processing tools (TOPUP and EDDY). We show that using this framework, it is possible to distinguish between good and bad quality datasets and, importantly, identify subsets of the data that may need careful visual inspection. We hope this QC tool will help harmonisation efforts across sites/studies.
Introduction
Diffusion MRI (dMRI) data can be affected by hardware and subject-related artefacts that can significantly bias downstream analyses. Therefore, automated QC is of great importance to detect data acquisition and pre-processing issues. This is especially critical in large population studies, where manual QC is not practical. In this abstract, we present an automated dMRI QC framework based on the FSL EDDY tool1. QC both at the subject level and the group level is performed using two different tools. QUAD (QUality Assessment for DMRI) generates single subject reports and stores the QC indices for each subject. SQUAD (Study QUality Assessment for DMRI) reads all the single subject outputs from QUAD, generates study-wise reports and, optionally, enters these into a QC-index database. Moreover, SQUAD can optionally update single subject reports, indicating how the subject’s dataset compares to other data, using either a study-specific group database or a pre-generated database obtained from a different dataset. Lastly, SQUAD also allows to report QC indices based on user-provided grouping variables.Methods
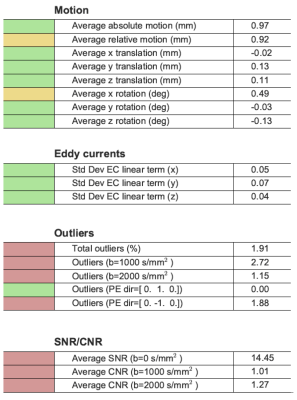
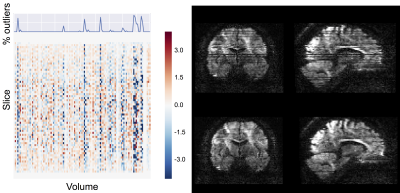
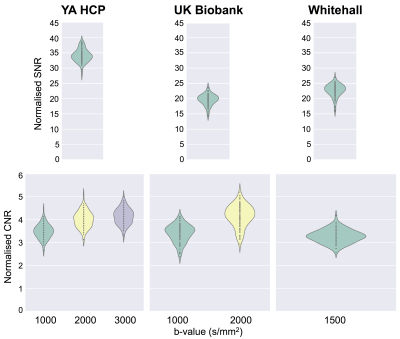
We ran the QC tools on a random set of 100 healthy subjects from three large imaging population studies: the young adults HCP2, UK Biobank3 and Whitehall4. The dMRI data collected within these studies cover a range of acquisition parameters (e.g. high vs low resolution or single vs multi-shell). To pre-process the dMRI data and derive QC indices, we use the comprehensive EDDY framework, which is based on a Gaussian process data predictor5. The tool can simultaneously estimate subject motion and eddy currents. The proposed QC framework uses the estimated motion parameters to detect problematic datasets. Moreover, it also computes the standard deviations of the estimated eddy currents’ linear terms. This is a good overall measure of the severity of eddy currents. Using the predicted data, EDDY can detect and replace outlier slices (due to motion-related signal dropout)6. The QC tool stores the number of outlier slices for each acquired volume. This is a useful QC metric when deciding whether or not to discard a specific volume. Moreover, the number of outliers per b-shell and per phase encoding direction can potentially be used to detect reconstruction issues or hardware faults. Using the EDDY-predicted data, it is also possible to estimate signal-to-noise (SNR) and contrast-to-noise ratio (CNR) measures. SNR is computed as the ratio between the average predicted b0 signal and the standard deviation of the residuals. CNR is computed as the ratio between the standard deviations of the predicted diffusion-weighted signal and of the residuals. For a sufficient sampling of q-space, this can be used to assess whether the data has enough angular contrast to estimate complex fibre geometries. Fig. 1 gives an overview of all the estimated QC indices. In addition to these quantitative indices, the tool also generates qualitative visual summaries of data quality to facilitate visual inspection (e.g. Fig 3).Results
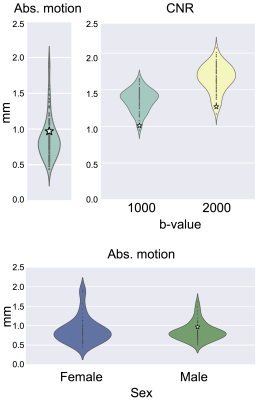
Fig. 1 shows an example summary table from a single Biobank subject generated using QUAD. The report has been updated using SQUAD to flag the QC metrics using a traffic-light colour scheme. This was evaluated against the performance of the whole sampled Biobank population. Fig. 2 shows the distributions of a selection of individual QC indices across the Biobank population. When using a grouping variable, each distribution is grouped to highlight potential significant differences in specific QC indices between two or more groups. Fig. 3 shows how outlier detection and replacement performances can be assessed using the output from QUAD. In each single-subject report, the volume-wise percentage of outliers is overlaid on top of a matrix containing the number of standard deviations between the slice mean and the average slice mean6. Fig. 4 shows a comparison of normalised CNR and SNR distributions between the different databases generated using SQUAD.Discussion and conclusions
We have presented a novel automated QC framework for dMRI data based on the output of FSL EDDY. Several quantitative metrics are generated to, e.g. help the user make decisions on whether to keep or discard volumes/subjects, or check if QC measures correlate with variables of interest such as group membership or disease state. Our tools can be used both at the single subject and at the group level to generate QC reports and databases. Any database generated by SQUAD can then be used to update single subject QC reports, thus putting individual subjects’ data in the context of a group to help identify potential issues at the individual level. Additionally, group QC reports can serve as baseline when evaluating new acquisition protocols, thus facilitating harmonisation efforts across studies, scanners, or sites.Acknowledgements
MB and SPF are supported by the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013/ ERC Grant Agreement no. 319456). JA is supported by the Wellcome-Trust Strategic Award 098369/Z/12/Z and by the NIH Human Connectome Project (1U01MH109589-01 and 1U01AG052564-01). MC is supported by the EPSRC UK (EP/L023067). FAA and UK Biobank brain imaging are funded by the UK Medical Research Council and the Wellcome Trust. SS is supported by the MRC UK (G1001354; ClinicalTrials.gov identifier: NCT03335696; PI: Ebmeier). SJ is supported by the MRC UK (Grant Ref: MR/L009013/1). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z).References
1. Andersson, J. L. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063-1078 (2016).
2. Sotiropoulos, S. N. et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage 80, 125-143, doi:10.1016/j.neuroimage.2013.05.057 (2013).
3. Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19, 1523-1536, doi:10.1038/nn.4393 (2016).
4. Filippini, N. et al. Study protocol: The Whitehall II imaging sub-study. BMC Psychiatry 14, 159, doi:10.1186/1471-244X-14-159 (2014).
5. Andersson, J. L. & Sotiropoulos, S. N. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. Neuroimage 122, 166-176 (2015).
6. Andersson, J. L., Graham, M. S., Zsoldos, E. & Sotiropoulos, S. N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 141, 556-572 (2016).
Figures