5330
Simultaneous IVIM and kurtosis acquisition in brain tumours: a trace-based protocol validated in a hybrid MR-PET system1INM4 - FZJ, Jülich, Germany, 2Instituto de Biofísica e Engenharia Biomédica, Sciences Faculty, University of Lisbon, Lisbon, Portugal
Synopsis
Diffusion weighted imaging such as intravoxel incoherent motion (IVIM) and non-Gaussian diffusion (NG-diff) allow the non-invasive study of tissue perfusion and are able to infer the organisation of the microstructure, respectively. Both are highly relevant in pathology characterisation, but are often acquired separately. Here we propose a unified IVIM/NG-diff protocol and apply it to brain tumour patients undergoing preoperative or follow-up scans in a hybrid MR-PET environment. The results agree well with previously reported values using standard techniques. Furthermore, indication for tumour grading power of IVIM parameters was found.
Purpose
Probing diffusion in distinct diffusivity ranges highlights different tissue properties. Intravoxel incoherent motion (IVIM) drives signal changes in the fast diffusivity regime and, characterized by parameters like perfusion fraction (f) and pseudo-diffusion coefficient (D*), is closely related to perfusion1. In an intermediate diffusivity range, diffusion in tissue can be described as being Gaussian, with the apparent diffusion coefficient (Dapp) reflecting tissue cellularity2. When probing slower diffusion ranges, water in brain tissue displays non-Gaussian diffusion, which can be described in a first step using the apparent kurtosis coefficient (Kapp)3. Acquisition protocols have been previously optimised for IVIM and NG-diff separately, and, if combined, they would be too lengthy for a clinical environment4. In this work we present a fast combined IVIM/NG-diff protocol designed to extract IVIM and kurtosis parameters in a single scan applied to a brain tumour patient cohort. A separate 30-direction 2-b value diffusion kurtosis acquisition (‘standard DKI’) was used to validate the kurtosis information provided by the IVIM/NG-diff protocol.Materials & Methods
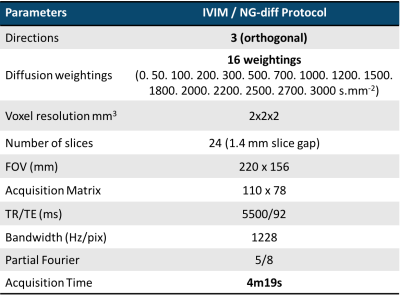
A cohort of 38 brain tumour patients was considered in this study (23 female, mean±std age 49,8 years old ± 14,42). Histological evaluation was carried out on 11 patients. Prior to scanning, written informed consent was given by the patients. The patients underwent simultaneous PET and MRI measurements acquired in a hybrid Siemens scanner based on a 3T Tim-TRIO MR system with a BrainPET insert. The MRI dataset consisted of standard clinical protocols, such as T1, T1C, T2, FLAIR and diffusion kurtosis imaging, and additional quantitative MRI scans including IVIM/NG-diff, the imaging parameters of which shown are in Table I. Data from the proposed protocol were denoised using a multiscale PCA-based denoising algorithm described in 5. The data were smoothed with a Gaussian filter to further increase SNR and sequentially fit to the following model:
$$S_b/S_0=f.e^{-b.D*}+(1-f).e^{-b.D_{app}+1/6.b^2.D_{app}^2.K_{app}}$$
where the first term characterises IVIM and the second NG-diffusion. From the denoised data set, only relevant b-values (0-2000s/mm2) were included in the fit. The multiexponential fit was performed sequentially. The first step, a non-linear fit of the NG-diffusion regime (b>800s/mm2, negligible IVIM contribution), delivered Dapp and Kapp coefficients; f and D* were calculated from the slope of the signal curve for b<200s/mm2. For the assessment of the performance of this new protocol and characterisation of different regions of interest, several masks were generated. Using the DKI data from the standard protocol, fractional anisotropy (FA) maps were estimated, from which low FA (0.05<FA<0.3) and high FA (FA>0.3) masks were generated. Active tumour tissue was identified from the dynamic 18F-Fluoro-ethyltyrosine (18F-FET) positron emission tomography (PET) scans6. Oedema masks were obtained using the ANTsR framework7.
Results & Discussion
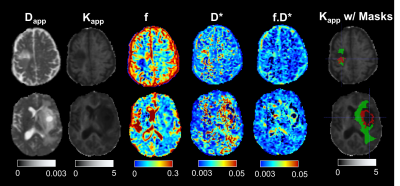
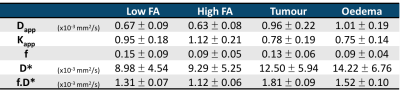
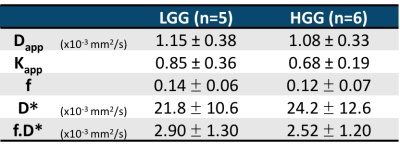
Histological evaluation of tumour tissue from primary patients determined 5 to have low grade glioma (LGG) and 6 to have high grade glioma (HGG). Figure 1 shows maps of 5 parameters estimated from the proposed protocol, on one LGG patient (upper row) and one HGG patient (bottom row). The right-most column shows the tumour and oedema masks overlaid to the Kapp maps of each subject. Table II summarises the mean±standard deviation values obtained for all the metrics across all subjects. The Dapp and Kapp obtained from this protocol were compared to their homologous tensor formulations (mean diffusivity and mean kurtosis) and showed good agreement8. The IVIM values reflect those reported in the literature9 (Table II). For low FA tissues (mainly grey matter), the capillary density is higher than in high FA tissues (white matter)10, which is seen here with higher f values in the low FA voxels. In oedema, the excess water in the tissue may result from inflammatory processes11. Hence the excess fluid leads to lower f and higher D*, as evidenced in table II. In tumour tissue, vascularisation and perfusion are highly heterogeneous, given the different types of tumour12. IVIM parameters are therefore expected to differ considerably depending on tumour type, stage and region, hinting towards tumour grading, supported by indications for tumour grading power based on both f and D* parameters (Mann-Whitney test, p<0.01) (Table III). Nevertheless, given the low sample size of primary patients in our study, assessment of larger cohorts is necessary.Conclusion
Here we present a protocol for simultaneous IVIM/NG-diff acquisition. Non-gaussian diffusion metrics obtained from the proposed protocol were in good agreement with those obtained using standard DKI derived metrics. We showed that the IVIM parameters obtained from our protocol are reflective of tissue physiology and are close to those in the literature. Furthermore, indication of tumour grading power was found using the IVIM parameters, although the limited sample size precludes firm conclusions.Acknowledgements
This work is funded in part by the Helmholtz Alliance ICEMED - Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Network Fund of the Helmholtz Association.
The authors would like to thank Claire Rick for the improvement in the quality of the written English.
References
[1] Le Bihan et al., “Separation of Diffusion and Perfusion in Intravoxel Incoherent Motion MR Imaging”, Radiology, 168, 496-505, 1988.
[2] Chen et al., “The correlation between apparent diffusion coefficient and tumor cellularity in patients: A meta-analysis”, PLoS ONE, 8(11), 2013.
[3] Jensen et al., “Diffusional kurtosis imaging: The quantification of non-Gaussian water diffusion by means of magnetic resonance imaging,” MRM, 53(6), 1432–1440, 2005.
[4] Finkenstaedt et al., “The IVIM signal in the healthy cerebral gray matter: A play of spherical and non-spherical components”, Neuroimage, 152, 340-347, 2017
[5] Oros-Peusquens et al., “Methods for molecular imaging of brain tumours in a hybrid MR-PET context: Water content, T2∗, diffusion indices and FET-PET”, Methods, 130, 135-151, 2017.
[6] Pauleit et al., “O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas,”Brain, 128(3), 678–687, 2005.
[7] Tustison et al., “Optimal Symmetric Multimodal Templates and Concatenated Random Forests for Supervised Brain Tumor Segmentation (Simplified) with ANTsR,” Neuroinformatics, 13(2), 209–225, 2015.
[8] Loução et al.., “A trace-based protocol for determining apparent kurtosis validated in a hybrid MR-PET clinical environment”, ISMRM 2017, 22nd -27th April 2017
[9] Federau et al., “Perfusion measurement in brain gliomas with intravoxel incoherent motion MRI”, AJNR, 35(2), 256-262, 2014
[10] Craigie “The arquitecture of cerebral capillary bed”, Biological Reviews, 20, 133–146, 1945
[11] Stokum et al., “Molecular pathophysiology of cerebral edema”, JCBFM, 36(3), 513-538, 2015
[12] Jain et al., “Angiogenesis in brain tumours”, Nature Neuro Reviews, 8, 610-622, 1007
Figures