5327
Efficient mutli-slice reduced field of view cardiac T2-ADC mapping with a restore pulse1Department of Radiological Sciences, University of California, Los Angeles, CA, Los Angeles, CA, United States, 2Biomedical Physics Interdepartmental Program, University of California, Los Angeles, CA., Los Angeles, CA, United States
Synopsis
Slice-following significantly reduces the scan time in cardiac diffusion weighting imaging by enabling free-breathing acquisition compatible with multi-slice coverage. However reduced field of view techniques result in a significant SNR penalty when combined with this multi-slice strategy. The objective of this work was to develop and evaluate a reduce field of view T2+ADC protocol compatible with multi-slice acquisition by using a “restore” pulse to improve higher SNR-efficiency.
Purpose
Simultaneous T2 and apparent diffusion coefficient (ADC) mapping enables acquiring co-registered information regarding myocardial edema (increased T2) and the degree of diffuse and/or focal myocardial fibrosis (increased ADC)1. Cardiac diffusion weighting imaging (cDWI) uses single-shot Spin-Echo EPI (SE-EPI) and second order motion compensated diffusion gradient2 to minimize cardiac bulk motion. Also due to the large number of images, navigator based strategies like slice-following3 (SF-NAV) can significantly reduce the scan time by enabling free-breathing acquisition with a 100% respiratory acquisition efficiency compatible with multi-slice (MS) heart coverage. SE-EPI permits fast image acquisition, but is susceptible to strong distortion artifacts along the lung/heart interface. These distortions can be minimized by applying a refocusing pulse in the phase encoding direction (orthogonal to the excitation pulse) to generate a reduced field-of-view (rFOV). However this rFOV SS-EPI approach is not compatible with MS acquisitions without incurring a significant SNR penalty.
The objective of this work was to develop and evaluate a rFOV T2+ADC mapping technique compatible with the MS SF-NAV approach to substantially decrease acquisition times. To do so, an additional refocusing RF pulse was added after the SE-EPI readout4 to “restore” the z-magnetization outside of the slice that was inverted by the rFOV process, thereby driving it to equilibrium and mitigating the SNR loss for a multi-slice interleaved acquisition. We hypothesize that a highest SNR-efficiency can be reach with a MS-rFOV+RESTORE protocols compare to traditional single-slice protocols.
Materials and Methods
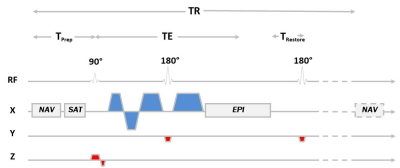
Sequence Design –A cDWI SE-EPI sequence was modified to support the following (Figure 1):
1) A cross-pair navigator was added before the acquisition to enable SF-NAV [2].
2) rFOV was realized by playing the slice-selection gradient of the refocusing pulse along the phase axis (orthogonal to excitation).
3) The Convex Optimized Diffusion Encoding (CODE) framework [3] was used to design motion compensated (M1=M2=0) and time optimal diffusion encoding gradients (CODE-M1M2).
4) The 180° restore pulse was added after the EPI readout [4] to restore the z-magnetization and enable multi-slice SF-NAV rFOV acquisitions.
MRI Experiments – On a 3T scanner (Prisma, Siemens), CODE-M1M2 diffusion encoding was used to acquire joint T2+ADC maps with 2.3x2.3x5.0mm resolution. Reference (b-value=0) images were obtained at two TEs (TE1=22ms with two averages, TE2=59ms with ten averages) and b-value=350s/mm2 images were obtained at TE=59ms using six diffusion directions and eight averages. SNR was calculated from the repeated b-value=0 images at TE=59ms. The restore pulse and rFOV techniques were combined in a slice-following strategy to enable T2+ADC acquisition in substantially reduced acquisition times. Reduced FOV multi-slice protocols with and without restore pulse (MS-rFOV+RESTORE and MS-rFOV) were evaluated and compared to a full magnetization recovery (TR>>T1) single-slice protocol (SS-rFOV) in a phantom and in healthy volunteers. Parameters for the three acquisitions were reported in Table 1.
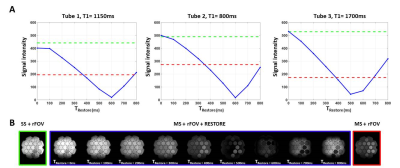
The signal recovery offered by the restore pulse is function of the T1 of the tissue, the number of slices and the time TRestore at which the pulse is played. Signal intensity as a function of TRestore was measured on a phantom with a range of T1 from 315ms to 2500ms.
Healthy volunteers (N=6) were imaged at mid-systole (TD=100ms) under free-breathing conditions using navigator based slice following3 (tracking factor=0.6). SNR, SNR-efficiency (SNR/√(Scan time per slice)), T2, and ADC were compared as described in 1 after manual segmentation of the LV and automatic rejection of corrupted signals5.
Results
Phantom acquisitions using MS-rFOV, SS-rFOV, and MS-rFOV+RESTORE protocols with different TRestore are shown in Figure 2. For the range of T1 present in the phantom, the signal regained by the restore pulse is maximum when TRestore is minimum and nearly equivalent to the signal from full recovery (SS-rFOV). However, due to the peripheral nerve stimulation limit after the EPI readout, a cooling time is required and the minimum TRestore achievable was only 200ms.
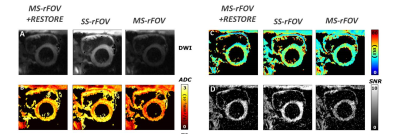
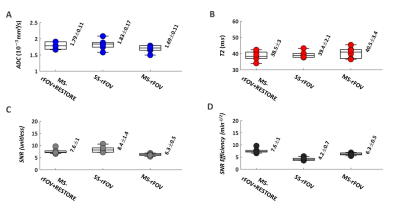
Figure 3 shows an example of DWI, T2, and ADC maps of a basal short-axis cardiac view acquired from a healthy volunteer. T2, ADC, SNR and SNR-efficiency distributions across volunteers are reported in Figure 4. No statistical differences were found for ADC or T2 between MS-rFOV, SS-rFOV, and MS-rFOV+RESTORE protocols. The highest SNR was obtained for SS-rFOV and MS-rFOV+RESTORE compared to MS-rFOV. Overall the best SNR-efficiency per slice was obtained with MS-rFOV+RESTORE (7.6±1 min-1/2) compared to MS-rFOV (6.2±0.5 min-1/2) and SS-rFOV (4.2±0.7 min-1/2).
Conclusion
The MS-rFOV+RESTORE technique
combined with slice following enables T2+ADC acquisition
compatible with clinical scan time constraints (~1 minute/slice) with the best
SNR-efficiency compared to MS-rFOV
and SS-rFOV.Acknowledgements
NIH/NHLBI R01 HL131823.References
[1] Aliotta E, Moulin K, Zhang Z., Ennis D. Simultaneous measurement of T2 and apparent diffusion coefficient (T2 +ADC) in the heart with motion-compensated spin echo diffusion-weighted imaging. Magnetic Resonance in Medicine. 2017.
[2] Aliotta E, Wu H, Ennis D. Convex Optimized Diffusion Encoding (CODE) Gradient Waveforms for Minimum Echo Time and Bulk Motion Compensated Diffusion Weighted MRI. Magnetic Resonance in Medicine. 2016.
[3] Moulin K, Croisille P, Feiweier T, Delattre BM, Wei H, Robert B, Beuf O, Viallon M. In vivo free-breathing DTI and IVIM of the whole human heart using a real-time slice-followed SE-EPI navigator-based sequence: A reproducibility study in healthy volunteers. Magnetic Resonance in Medicine. 2016.
[4] Eun-Kee J, Seong-Eun , Junyu G, Eugene G. K., and Dennis L. P. High-Resolution DTI with 2D Interleaved Multislice Reduced FOV Single-Shot Diffusion-Weighted EPI (2Dss-rFOV-DWEPI). Magnetic Resonance in Medicine. 2005.
[5] Aliotta E, Rapacchi S, Hu P, Ennis D, editors. Increased maximum gradient amplitude improves robustness of spin-echo cardiac diffusion-weighted MRI. SCMR; 2015; Nice, Fr.: JCMR.
Figures