5324
Improved signal-to-noise ratio with highly asymmetric STEAM-EPI (HASTEAM-EPI)1Brain Imaging Center (BIC), Goethe University Frankfurt, Frankfurt am Main, Germany
Synopsis
Several methods exist for increasing the signal-to-noise ratio (SNR) in high spatial resolution imaging, e.g., by using reduced field-of-view techniques or reduced echo-times (TE). Here, a novel module dubbed “highly asymmetric STEAM" (HASTEAM) is proposed, where the stimulated echo (STE) occurs prior to the EPI readout and thus maintains a constant duration of STE preparation, independent of the acquisition matrix-size. This shortens TE and allows for higher SNR, in particular for high spatial resolutions. SNR gain of HASTEAM over the standard STEAM is simulated and compared to in vivo results for different resolutions in structural and diffusion-weighted imaging.
Introduction
Structural and diffusion-weighted imaging (SI, DWI) in MRI require high spatial resolution and signal-to-noise ratio (SNR). Over the last few decades, several methods were introduced to increase the SNR, e.g., by using the reduced field-of-view (FOV) technique or a shortened echo time (TE). The diffusion-weighted stimulated echo acquisition mode (STEAM) [1] allows for long diffusion times, which is advantageous for achieving high b-values or for assessing restricted diffusion at short TE, e.g., to investigate tissue with short T2). Improvements of diffusion-weighted STEAM via correction for eddy currents [2] and optimization of slice profiles [3] have been reported. In the standard implementation of STEAM, the occurrence of the stimulated-echo (STE) coincides with central k-space sampling during the echo planar imaging (EPI) readout, thus prolonging TE with increasing spatial resolution. In this study, a novel module dubbed “highly asymmetric STEAM" (HASTEAM) is proposed, where the STE occurs prior to the EPI readout, thus maintaining a constant duration of the STE preparation (i.e., independent of any spatial resolutions). The goal was to determine the SNR gain of the proposed HASTEAM module over the standard STEAM for different resolutions.Methods
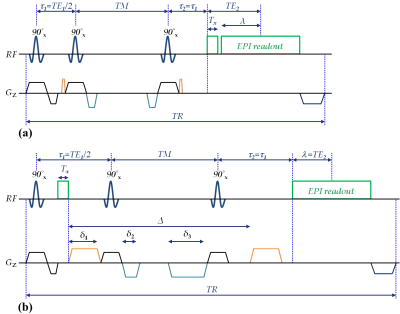
Sequence: Figure 1 shows a schematic representation of the proposed HASTEAM-EPI sequence for SI (a) and DWI (b). Please note that the acquisition of navigator echoes is performed before image acquisition in SI or before the diffusion-weighted gradients in DWI. In the HASTEAM preparation, the STE forms at TE1 with T1- and T2-weighting during TM and TE1, respectively. In addition, there is a T2* decay during TE2 which depends on the acquisition matrix-size. Thus, the signal intensity is given by 0.5[exp(-TE1/T2).exp(-TM/T1).exp(-TE2/T2*)]. In contrast conventional STEAM-EPI requires an additional delay λ=TE2 within τ1 in order to form a STE at the center of the readout, thus yielding a signal intensity of 0.5[exp(-TE1/T2).exp(-TM/T1).exp(-2TE2/T2)]. Therefore, the HASTEAM-EPI sequence should yield a higher SNR for TE2/T2*<2TE2/T2 or: T2*>T2/2.
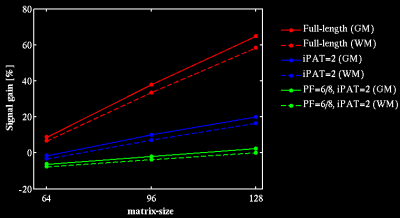
Simulation: Signal gains of HASTEAM-EPI versus STEAM-EPI were calculated for different sampling schemes affecting the EPI readout duration: matrix-size=[64,96,128], echo-spacing=0.54/0.82/0.86ms, full EPI readout vs. EPI readout reduction via 2-fold acceleration (iPAT=2) with/without 75% partial Fourier (PF) encoding. Timing parameters were TE1=10ms and TM=60ms. The relaxation times T2=60/65ms [4], T2*=45/52ms and T1=832/1331ms [5] were assumed for white and gray matter (WM, GM), respectively.
Protocols: Two DWI (in-plane resolutions: 2mm/1.5mm, matrix-size: 96/128) and three SI protocols (in-plane resolutions: 3mm/2mm/1.5mm, matrix-size: 64/96/128) were used with full or reduced (iPAT=2) EPI readout, FOV=192×192mm2, 60 axial slices (slice-thickness=2mm, no gap), TE1=10/48ms (SI/DWI), TR per volume=9/15/16s for SI and 15/18s for DWI.
Experiments: Four healthy volunteers (2 females and 2 males, mean age ± SD: 23.5±3.5 years) were scanned on a 3T whole-body MRI-scanner (MAGNETOM Trio, Siemens Healthcare, Erlangen, Germany), using a body-TX coil and an 8-channel phased-array head-RX coil and the above protocols. Written informed consent was obtained from all participants before scanning.
Results
Simulation: Figure 2 shows that HASTEAM-EPI yields more signal than standard STEAM-EPI, both for WM and GM. Particularly, signal gains are high for large matrix-sizes and fully sampled EPI readout.
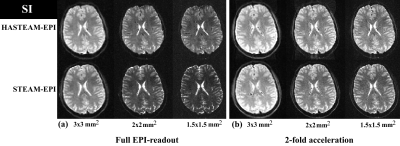
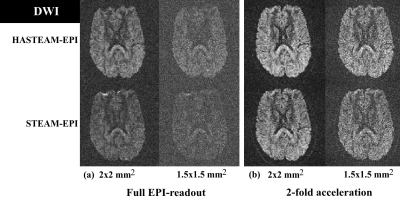
Experiments: Figure 3 shows in vivo results for the different SI protocols. For an in-plane resolution of 3mm, HASTEAM-EPI yielded signal gains in corpus callosum/putamen of 18%/10% for full EPI readout (Fig. 3a), but no gain for iPAT=2 (Fig. 3b). For higher in-plane resolutions, HASTEAM-EPI always yielded a positive signal gain. As an example, for an in-plane resolution of 1.5mm, the respective values were 46%/51% and 17%/29%. Figure 4 shows in vivo results for the different DWI protocols. For an in-plane resolution of 2mm, signal gains in the corpus callosum were 21% (Fig. 4a, full EPI readout) and 4.3% (Fig. 4b, iPAT=2). For an in-plane resolution of 1.5mm, the respective gains were 13.4% and 16.6%. Signal gains in SI and DWI differ mainly due to the difference in TE1.
Discussion/Conclusion
Simulations and experiments show that HASTEAM may yield an increased SNR due to the constant duration of the STE preparation, independent of the EPI readout. Differences in predicted (simulation) and measured (in vivo experiments) signal gains may be due to deviations of real from assumed T2/T2* values in WM and GM. Signal gains were lower in DWI but may be increased by shortening TE1, e.g., via stronger DWG or separate acquisition of navigator echoes. Signal gains may increase at higher magnetic field strengths, due to shortened T2 and T2*.Acknowledgements
No acknowledgement found.References
1. Tanner JE (1970). Use of the stimulated echo in NMR diffusion studies. J Chem Phys 52(5), 2523-2526.
2. Steidle G, Schick F (2006). Echoplanar diffusion tensor imaging of the lower leg musculature using eddy current nulled stimulated echo preparation. Magn Reson Med 55(3), 541-548.
3. Shrestha et al. (2016). Optimization of slice profiles for diffusion-weighted STEAM imaging. In: Proceedings of the 33rd Annual Scientific Meeting of ESMRMB, Vienna, p 159-160.
4. Nöth et al. (2017). Quantitative in Vivo T2 Mapping Using Fast Spin Echo Techniques-A Linear Correction Procedure. NeuroImage 157, 476-485.
5. Wansapura et al. (1999). NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imag 9(4), 531-538.
Figures