5321
Multiband Diffusion-Weighted MRI of the Eye and Orbit Free of Geometric Distortions Using a RARE-EPI Hybrid1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 3Department of Ophthalmology, University of Rostock, Rostock, Germany, 4Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
This study shows that multiband diffusion-weighted RARE-EPI has the capability to acquire distortion-free images of the eye and orbit with ample diffusion contrast for slices in close proximity. The results underpin the challenges of ocular imaging at 3.0 T and 7.0 T for echo planar imaging and demonstrate that these issues can be offset by using accelerated RARE based approaches. This benefit can be exploited for the assessment of spatial arrangements of the eye segments and their masses with the goal to provide guidance in diagnostic assessment and treatment of ophthalmological diseases.
INTRODUCTION
Diffusion-weighted imaging (DWI) provides information about tissue microstructure1,2. Single-shot echo planar imaging (EPI) is the most common technique used for DWI applications in the brain but is prone to geometric distortions and signal voids which is detrimental for ocular imaging3,4. Rapid Acquisition with Relaxation Enhancement imaging (RARE) presents a valuable alternative for DWI with high anatomic accuracy5-7. DWI-RARE has recently been suggested for ocular imaging free of geometric distortions8. For accelerated DWI of the eye this work proposes a multiband (MB) multi-shot diffusion-weighted RARE-EPI hybrid pulse sequence9,10, joining the anatomical integrity of RARE with the imaging speed and radiofrequency (RF) power deposition advantage of EPI. The applicability for DWI of the eye free of geometric distortions as well as the separability of the aliased slices is validated in phantom studies and demonstrated in a volunteer study including healthy subjects and a patient with an arachnoidal cyst.METHODS
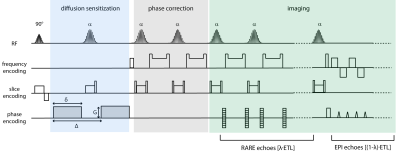
The diffusion-weighted RARE-EPI (DW-RARE-EPI) variant is shown schematically in Figure 1. [(1-λ)×ETL] RARE echoes are replaced by EPI echoes (ETL=echo train length) with (0≤λ≤1)9. For multiband imaging two Hanning filtered SINC pulses were added and CAIPIRINHA encoding along the slice direction was employed to facilitate slice separation11. A constant phase of 90° was applied for slice 2 to reduce the peak power of the multiband RF pulse12. Morphological and diffusion-weighted images for apparent diffusion coefficient (ADC) mapping were acquired at 3.0 T (Magnetom Verio, Siemens, Erlangen, Germany) for a cylindrical structure phantom and in-vivo for two brain slices separated by a 1 cm gap and covering the eyes. For comparison, three alternative diffusion-weighted pulse techniques were employed: (i) MB multi-shot RARE (ms-RARE)8 that was recently proposed for diffusion-weighted imaging free of geometric distortions, which is identical to DW-RARE-EPI with λ=1, (ii) single-shot EPI (ss-EPI) as the clinical standard for DWI and (iii) readout-segmented EPI (rs-EPI) as a sophisticated EPI variant13.RESULTS
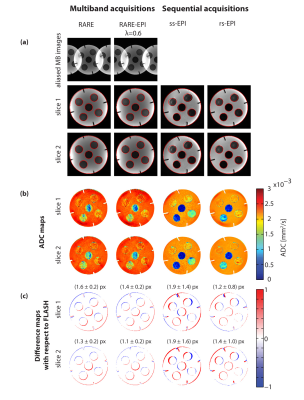
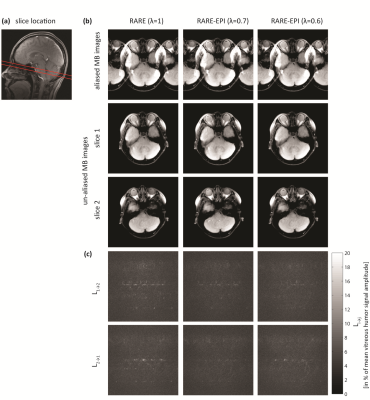
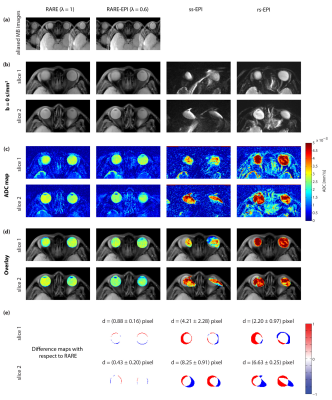
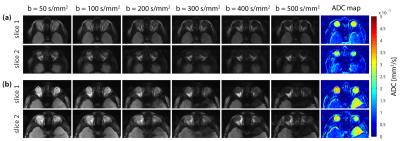
Figure 2 summarizes the results of the multiband DWI phantom study. ss-EPI and rs-EPI showed increased geometric distortions in comparison to RARE and RARE-EPI. The distortions obtained for slice 1 and slice 2 were in the same range for all approaches. ADC values determined for the distilled water compartment of the phantom were comparable for both slices, meaning that the multiband pulses do not generate any detrimental effect on the DWI technique. The results obtained for morphological simultaneous multi-slice RARE-EPI imaging are summarized in Figure 3. The location of the two selected slices displaced by 1 cm is illustrated in Figure 3a. Aliased and un-aliased images are shown in Figure 3b together with normalized leakage values Li→j (Figure 3c)14,15. The unaliased MB images for slice 1 and slice 2 demonstrate successful slice separation for the applied range of λ-values. The quantitative analysis of signal leakage from slice 1 into slice 2 and vice versa revealed values of 8.5%/8.3% () for λ=1, 7.5%/7.5% for λ=0.7 and 7.9%/8.1% for λ=0.6. Figure 4 illustrates the immunity of multiband DW-RARE-EPI ADC mapping to geometric distortions. This manifests itself in the point-spread-function induced displacement of the center of gravity of both eye balls of less than one pixel for RARE-EPI with respect to ms-RARE. Diffusion-weighted simultaneous multi-slice RARE-EPI (λ=0.6) data and the corresponding ADC maps are displayed in Figure 5 for a healthy volunteer and a subject with an arachnoidal cyst. Successful slice separation was achieved for the complete range of applied b‑values using a non-diffusion-weighted calibration data set with half resolution compared to the multiband data.DISCUSSION
Anatomical integrity of multiband RARE-EPI was demonstrated and quantified in phantom imaging and for in-vivo diffusion-weighted acquisitions. The results indicate that half of the RARE echoes in the echo train can be replaced by EPI echoes while maintaining anatomical accuracy. The close location of the simultaneously acquired slices compared to the slice thickness of 5 mm is of particular importance for ophthalmic imaging due to the small size of the eye as the target organ. It is a recognized limitation to our feasibility study that the multiband factor was limited to 2. Replacing the current RF pulses by PINS pulses may be a promising candidate to increase the number of simultaneous acquired slices16-18.CONCLUSION
This study shows that diffusion-weighted multiband RARE-EPI has the capability to acquire high fidelity, distortion-free images of the eye and the orbit for two simultaneous slices. It is shown that RARE-EPI maintains the immunity to B0 inhomogeneities reported for RARE imaging. This benefit can be exploited for the assessment of ocular masses and pathologic changes of the eye and the orbit.Acknowledgements
The authors wish to thank Steen Moeller (Department of Radiology and Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, Minnesota, USA) for providing the slice GRAPPA algorithm used for our data analysis.References
1. Norris DG, Niendorf T, Leibfritz D. Healthy and infarcted brain tissues studied at short diffusion times: The origins of apparent restriction and the reduction in apparent diffusion coefficient. NMR Biomed. 1994;7(7):304-310.
2. Niendorf T, Dijkhuizen RM, Norris DG, et al. Biexponential diffusion attenuation in various states of brain tissue: Implications for diffusion-weighted imaging. Magn Reson Med. 1996;36(6):847-857.
3. Erb-Eigner K, Willerding G, Taupitz M, et al. Diffusion-Weighted Imaging of Ocular Melanoma. Invest Radiol. 2013;48(10):702-707.
4. Xu X, Wang Y, Hu H, et al. Readout-segmented echo-planar diffusion-weighted imaging in the assessment of orbital tumors: comparison with conventional single-shot echo-planar imaging in image quality and diagnostic performance. Acta Radiol. 2017;doi: 10.1177/0284185117695667:284185117695667.
5. Hennig J, Nauerth A, Friedburg H. RARE imaging: A fast imaging method for clinical MR. Magn Reson Med. 1986;3(6):823-833.
6. Norris DG, Boernert P, Reese T, et al. On the application of ultra-fast rare experiments. Magn Reson Med. 1992;27(1):142-164.
7. Williams CFM, Redpath TW, Norris DG. A novel fast split-echo multi-shot diffusion-weighted MRI method using navigator echoes. Magn Reson Med. 1999;41(4):734-742.
8. Paul K, Graessl A, Rieger J, et al. Diffusion-Sensitized Ophthalmic MRI Free of Geometric Distortion at 3.0 T and 7.0 T: A Feasibility Study in Healthy Subjects and Patients with Intraocular Masses. Invest Radiol. 2015;50(5):309-321.
9. Hillenbrand C, Hahn D, Haase A, et al. MR CAT scan: a modular approach for hybrid imaging. MAGMA. 2000;10(3):183-199.
10. Paul K, Waiczies H, Kuehne A, et al. Accelerated Diffusion-Sensitized MR Imaging of the Eye and Orbit at 3.0 T and 7.0 T free of Geometric Distortions Using a Combined RARE-EPI Acquisition Technique. In Proc Intl Soc Mag Reson Med 2017. p.1096.
11. Breuer FA, Blaimer M, Heidemann RM, et al. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005;53(3):684-691.
12. Hennig J. Chemical shift imaging with phase-encoding RF pulses. Magn Reson Med. 1992;25(2):289-298.
13. Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009;62(2):468-475.
14. Xu J, Moeller S, Auerbach EJ, et al. Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage. 2013;83(991-1001.
15. Schmitter S, Moeller S, Wu X, et al. Simultaneous multislice imaging in dynamic cardiac MRI at 7T using parallel transmission. Magn Reson Med. 2017;77(3):1010-1020.
16. Norris DG, Koopmans PJ, Boyacioglu R, et al. Power Independent of Number of Slices (PINS) radiofrequency pulses for low-power simultaneous multislice excitation. Magn Reson Med. 2011;66(5):1234-1240.
17. Norris DG, Boyacioglu R, Schulz J, et al. Application of PINS radiofrequency pulses to reduce power deposition in RARE/turbo spin echo imaging of the human head. Magn Reson Med. 2014;71(1):44-49.
18. Gagoski BA, Bilgic B, Eichner C, et al. RARE/turbo spin echo imaging with Simultaneous Multislice Wave-CAIPI. Magn Reson Med. 2015;73(3):929-938.
Figures