5312
Alterations in functional brain network topology in Tourette’s syndrome1Institute of Neuroscience and Medicine - 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Aachen, Germany, 3JARA-BRAIN Translational Medicine, Aachen, Germany, 4TRIMAGE – consortium, Aachen, Germany, 5Institute of Neuroscience and Medicine - 11, INM-11, Forschungszentrum Juelich, Juelich, Germany, 6Department of Neurology, RWTH Aachen University, Aachen, Germany, 7Department of Electrical and Computer Systems Engineering, and Monash Biomedical Imaging, School of Psychological Sciences, Monash Institute of Medical Engineering, Monash University, Melbourne, Australia
Synopsis
Tourette syndrome (TS) is a neurodevelopmental disorder with typical onset in childhood. Its characteristic motor tics are said to be attributed to dysfunction in the cortico-striato-thalamo-cortical circuit and cerebellar communication. Brain functional connectivity along with network topology analysis provides a useful tool to understand communication strategies in the brain. Hence we aim to investigate alterations in functional and effective connectivity in brains of patients with TS. Based on prior results1,2, we hypothesize that connectivity of basal ganglia, thalamus and cerebellum with other regions will be altered.
Introduction
Tourette syndrome (TS) is a neurodevelopmental disorder characterized by multiple motor and at least one vocal or phonic tic2. It is often accompanied by comorbidities such as obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD) or depression3,4. Dysfunction of the cortico-striato-thalamo-cortical circuit is considered as core pathophysiology2,5–7. Investigations in pediatric TS have revealed altered functional brain topology in visual, default-mode, language and fronto-parietal areas8–10.
Except for one study on connectivity of the globus pallidus internus11, no studies have focused on connectivity of the basal ganglia and thalamus with other brain regions. Since these two regions are key players in the CSTC circuit, it is crucial to investigate the communication of these regions. Hence, the study aim is to specifically investigate the functional and effective connectivity of the basal ganglia, thalamus and cerebellum with the whole brain. Furthermore, the study aims at finding regions showing altered functional and effective graph topological properties.
Methods
Resting state fMRI data of 28 TS and 28 age-matched controls, recorded in a 3T MR system (Trio, Siemens, Germany) using an EPI protocol (TR 3200msec, TE 60ms, 12 minutes, eyes closed) were used for this study. This dataset with another analysis focus has been published in1. Structural MRI images were acquired using the MPRAGE protocol (TR 2200msec, TE 3.93msec, FA 15°, 176 slices, FOV 256 x 256 mm, voxel size 1mm isotropic). ROI-based Functional (correlation) and effective (regression) connectivity analyses of the basal ganglia, thalamus and cerebellum with the whole brain were performed after preprocessing (realignment, slice time correction, outlier scan detection, segmentation, normalization, de-noising using CompCor , temporal band pass filtering 0.008-0.09Hz) using the SPM based toolbox CONN v17.c12. The whole brain was parcellated into 132 regions as given by the atlas included in the toolbox CONN v17.c (Cortical and Subcortical ROIs from Harvard-Oxford Maximum Likelihood atlas, Cerebellar parcellation from AAL atlas). Out of this the basal ganglia comprised of 8 regions, thalamus 2 and cerebellum (including vermis) 25. Additionally, graph theory was applied to thresholded (>0.4 or <-0.4) connectivity matrices.Results
Significant alterations in functional and effective connectivity were observed (see figure 1 and figure 2).
Whole brain functional graph topology assessment showed increased global efficiency in left and right caudate, putamen, accumbens, pallidum, left thalamus, 3rd and 4th left cerebellar regions, and cerebellar vermis regions 12, 45, 6, 7, 8. Average path length was increased in the right pallidum and decreased in the right frontal orbital cortex. Other investigated node metrics such as local efficiency, betweenness centrality, cost, clustering coefficient and degree remained unaltered.
With the effective brain network, global efficiency was increased in the right accumbens, left and right pallidum, cerebellar vermis 3 and right inferior frontal gyrus (pars opercularis) and reduced in left cerebellar crus 1. Decrease in average path length was observed in the precuneus, posterior cingulate, left and right angular gyrus and insular cortex, left cuneal cortex, left central opercular cortex and right paracingulate gyrus. The clustering coefficient was increased in the right accumbens. Other metrics such as local efficiency, betweenness centrality, cost and degree remained unaltered.
Discussion
The caudate and putamen receive significant glutamatergic inputs from the cortex. Functional connectivity alterations between dorsal striatum and other cortical areas indicate a dysfunction in striato-cortical glutamatergic communication. Increased connectivity could mean excessive communication which is supported by spectroscopy findings on increased glutamate levels in striatum and premotor cortex2. As the cerebellum is involved in precision and recently has been found to be involved in higher cognitive function, reduced cerebellar connectivity could mean loss of precision and uncoordinated movement. Additionally, it could have implications on other cognitive functions which remains an open question13.
Increased global efficiency of striatal, pallidal, thalamic and cerebellar regions indicates increased speed of communication through these areas. This could indicate a lack of inhibition in these regions. This supports loss of striatal GABAergic inhibitory interneurons and decreased binding potential of GABA receptors in the ventral striatum, globus pallidus and thalamus previously shown in post mortem and PET studies2. The finding of an increased clustering coefficient in the right accumbens might reflect the biological substrate why tics increase in frequency and intensity during e.g. emotional distress.
Conclusion
Functional and Effective connectivity analyses revealed altered connectivity in the basal ganglia, thalamus and cerebellum. Graph topological changes were most prominent in parts of the default mode network, prefrontal areas (lack of inhibition), insular cortex (self-awareness), nucleus accumbens (emotional distress triggering tics) and the cerebellum (coordination, cognition).Acknowledgements
The authors would like to thank the German Tourette Association, all patients and their families for participation.References
1. Neuner, I. et al. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front. Hum. Neurosci. 8, 362 (2014).
2. Robertson, M. M. et al. Gilles de la Tourette syndrome. Nat. Rev. Dis. Prim. 3, 16097 (2017).
3. Khalifa, N. & von Knorring, A.-L. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Dev. Med. Child Neurol. 45, 315–319 (2003).
4. Cavanna, A. E., Servo, S., Monaco, F. & Robertson, M. M. The behavioral spectrum of Gilles de la Tourette syndrome. J. Neuropsychiatry Clin. Neurosci. 21, 13–23 (2009).
5. Leckman, J. F. Tourette’s syndrome. in Lancet 360, 1577–1586 (Elsevier, 2002).
6. Singer, H. S. Tourette’s syndrome: From behaviour to biology. Lancet Neurology 4, 149–159 (2005).
7. Schlemm, E. et al. Altered topology of structural brain networks in patients with Gilles de la Tourette syndrome. Sci. Rep. 7, 10606 (2017).
8. Greene, D. J. et al. Multivariate pattern classification of pediatric Tourette syndrome using functional connectivity MRI. Dev. Sci. 19, 581–598 (2016).
9. Wen, H. et al. Combining Disrupted and Discriminative Topological Properties of Functional Connectivity Networks as Neuroimaging Biomarkers for Accurate Diagnosis of Early Tourette Syndrome Children. Molecular Neurobiology 1–19 (2017). doi:10.1007/s12035-017-0519-1
10. Church, J. A. et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain 132, 225–238 (2009).
11. Ji, G.-J. et al. Globus Pallidus Interna in Tourette Syndrome: Decreased Local Activity and Disrupted Functional Connectivtiy. Front. Neuroanat. 10, 93 (2016).
12. Whitfield-Gabrieli, S. & Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2, 125–141 (2012).
13. Schmahmann, J. D. An Emerging Concept: The Cerebellar Contribution to Higher Function. Arch. Neurol. 48, 1178 (1991).
Figures
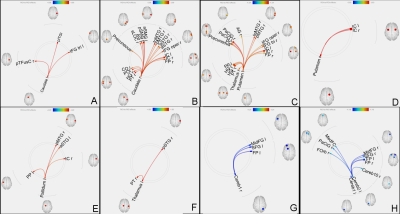
Increased functional connectivity of left caudate (A), right caudate (B), left putamen (C), right putamen (D), left pallidum (E) and left thalamus (F) with several other regions.
Reduced functional connectivity of right cerebellar crus 1 (G) and left cerebellar crus 2 (H) with other brain regions.
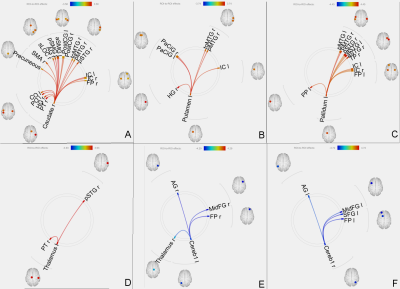
Increased effective connectivity of right caudate (A), left putamen (B), left pallidum (C) and left thalamus (D) with several other regions.
Reduced effective connectivity of left cerebellar crus 1 (E) and right cerebellar crus 1 (F) with other brain regions.