5311
Characteristic Changes of Shape in Subcortical Nuclei in Major Depressive Disorder1Department of Radiology, West China Hospital of Sichuan University, Huaxi MR Research Center (HMRRC), Chengdu, China, 2Sichuan University, West China School of Clinical Medicine, Chengdu, China, 3Sichuan University, West China School of Public Health, Chengdu, China
Synopsis
We analysis alterations of volume and shape of subcortical nuclei in a relatively large sample of adult patients with Major Depressive Disorder (MDD) using an automatically segmentation and vertex-based shape analysis protocol. We found that hippocampus, putamen and accumbens were impaired in patients with MDD and subregional shape of hippocampus, accumbens and pallidum may have potential predictive value of treatment response in patients with MDD. Shape analysis may provide more evidence of neuropathology related to depression from a different perspective. Future study should consider shape and volume analysis simultaneously.
Purpose
Major depressive disorder (MDD) is a debilitating disorder recognized by decreased motivation, hopelessness, loss of interest in pleasurable activities, thus widely speculated to be associated with reward system dysfunction [1]. Subcortical nuclei, including basal ganglia, thalamus, amygdala and hippocampus are critical components in reward system, however, previous volumetric studies yield out inconsistent results of alteration of subcortical nuclei in MDD patients [2,3]. Vertex-based shape analysis is a state-of-art three-dimentional analysis method with potential of higher sensitivity in detecting morphometry alteration[4]. Herein, in present study we aim to determine volume and shape alteration of subcortical structures in a relatively large sample of MDD patients.Method
71 DSM-IV criteria diagnosed MDD patients and 64 age and sex matched healthy control were recruited and informed consents were obtained from all subjects. Hamilton Depression Scale (HAMD) scores were collected before and after 6 weeks of medication treatment (referred as HAMD-pre and HAMD-post, respectively). Reduction Rate of HAMD score (RRS), defined as the ratio [(HAMD-pre) – (HAMD-post)/(HAMD-pre]] was calculated as behalf of treatment response. High resolution T1 weighted images were obtained before medication treatment using a volumetric 3-dimensional Spoiled Gradient Recall (SPGR) sequence (TR/TE = 8.5/3.4ms; flip angle = 12o; 156 axial slices with thickness = 1mm, field of view = 24×24cm2 and data matrix = 512×512) via a GE 3.0 T scanner.
Using FSL-FIRST software [4], 7 subcortical nuclei including accumbens, amygdala, putamen, pallidum, hippocampus and thalamus, left and right hemisphere respectively, were automatically segmented based on learned models. All segmentation was manually checked for software failure.
Statistic analysis of shape was further carried out via Randomise [5] program. Group difference and correlation between illness duration, HAMD-pre score, RRS and measurements of subcortical nuclei were examined with a general linear model (GLM) and Threshold-Free Cluster Enhancement (TFCE) was used for permutaion test. FEW and Monte Carlo method was used for multiple comparison correction of group differences and correlation analysis, respectively. Volume differences between two groups were compared using Analysis of Covariates (ANCOVA) with age as covariates in SPSS software.
Result
Demographic information for all subjects was shown in table1.
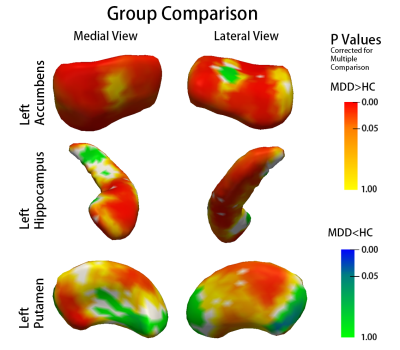
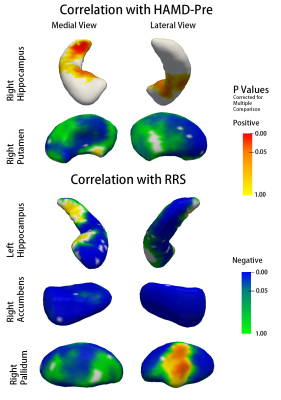
Shape analysis showed widespread subregional deformations in the form of expansion in patients with MDD in left accumbens, left putamen and left hippocampus, relative to HC (Fig 1). HAMD-Pre score was correlated with subregional shape of putamen negatively and medial hippocampal tail positively on the right hemisphere. RRS was negatively correlated with subregional shape of left hippocampus, right accumbens area and right pallidum (Fig 2).
Volumes of nearly all subcortical nuclei were slightly smaller in patients with MDD comparing with HC, however, none of differences between two groups reached statistical significance after adjusting for age (Table 2).
Discussion & Conclusion
The present study demonstrated subcortical nuclei, particularlyhippocampus, putamen and accumbens were implicated in MDD. Hippocampus, accumbens and putamen has an important role in reward processing, and depressed patients have been reported to show decreased activation to rewarding stimuli. Briefly, accumbens receives projects from orbitofrontal cortex (OFC), and putamen receives projects from sensorimotor cortex, and hippocampus as an important component of limbic system receives projects from anterior cingulate cortex (ACC) and dorsomedial prefrontal cortex (dmPFC). Taken together, they form the cortico-basal ganglia circuitry and was reported abnormal in activity in various psychiatric disroders, including MDD. More importantly, normalization of cerebral activity in these regionshas been associated with antidepressant treatment response[1].
Herein, our findings provide neural structural mechanism of abnormal reward system in patients with MDD. Furthermore, subregional shape deformation in left hippocampus, right accumbens and right pallidum may have potential predict value of medication treatment response as revealed by secondary correlation analysis.
Acknowledgements
No acknowledgement found.References
[1]Annu. Rev. Physiol. 2016.78:327-50
[2]Transl Psychiatry. 2017 May 2;7(5):e1116.
[3]Psychol Med. 2012 Apr;42(4):671-81.
[4]Neuroimage, 2011. 56(3): p. 907-22.
[5]NeuroImage, 2014;92:381-397.
Figures