5310
The effects of relapse on gray matter volume changes in patients with Major Depression – a longitudinal VBM studyHarald Kugel1, Dario Zaremba2, Katharina Dohm2, Ronny Redlich2, Dominik Grotegerd2, Robert Strojny2, Susanne Meinert2, Christian Buerger2, Verena Enneking2, Katharina Foerster2, Jonathan Repple2, Nils Opel2, Bernhard T Baune3, Pienie Zwitserlood4, Walter Heindel1, Volker Arolt2, and Udo Dannlowski2
1Department of Clinical Radiology, University of Muenster, Muenster, Germany, 2Department of Psychiatry, University of Muenster, Muenster, Germany, 3Discipline of Psychiatry, University of Adelaide, Adelaide, Australia, 4Department of Psychology, University of Muenster, Muenster, Germany
Synopsis
Structural brain alterations in major depressive disorder (MDD) are associated with patients' course of illness, especially in progressive and recurrent MDD. Here, a longitudinal study investigated the influence of relapse on gray matter volume. As a result, Voxel based morphometry showed a decrease of insular and DLPF gray matter volume in patients with at least one relapse, while volume in patients without relapse was stable. This illustrates the negative effect of relapse on structural brain alterations.
Introduction
With a lifetime prevalence of 16%, major depressive disorder (MDD) is one of the most common and debilitating psychiatric disorders 1. Although about 80% of the patients diagnosed with MDD experience recovery within two years, less than half of these patients remain symptom-free for two years following recovery 2. Relapse rates increase dramatically with subsequent episodes over time, leading to a high risk of chronicity and severe psychosocial consequences among patients 3. This poses the question about psychopathological mechanisms behind relapse to improve prognosis and maintenance treatment of MDD. MDD is associated with structural changes in specific brain areas, which are influenced by the course of illness, especially in progressive and recurrent MDD 4. Yet, longitudinal studies investigating the influence of relapse on gray matter volume changes are still too rare to warrant conclusive findings. The aim of this study was to investigate structural brain alterations in patients with MDD across the entire brain as a function of the course of illness during the follow-up interval.Methods
Sixty-four patients with MDD and 59 healthy controls underwent structural MRI at baseline and approximately two years later at follow-up. After exclusion of participants during preprocessing of data, the final sample consisted of 60 patients and 54 healthy controls. Depending on their course of illness between scans, patients were subdivided into 37 patients with relapse and 23 patients without relapse. MRI images were obtained at 3 T (Gyroscan Intera with Achieva upgrade) using a 3D T1w TFE-sequence (TR/TE/FA = 7.4 ms/3.4 ms/9°, with an inversion prepulse every 814.5 ms, reconstructed to voxels of .5 mm edge length). Images were processed using the pipeline of the CAT12-toolbox 5, by a longitudinal voxel-based morphometry approach, statistical analysis of gray matter was done in SPM12 6. Differences in gray matter volume were analyzed in a 3x2 ANCOVA with group (no relapse, relapse and healthy controls) and time (baseline, follow-up) using a full factorial design. Potential confounds of medication and depression severity on gray matter volume were assessed with correlation analysesResults
The ANCOVA showed a significant group by time interaction revealing that patients with at least one relapse between scans showed a decrease of right insular and right dorsolateral prefrontal cortex (DLFPC) gray matter volume. In patients without relapse, gray matter volume in these regions was stable. Healthy controls showed an increase. Volume changes over time were neither associated with psychiatric medication nor with depression severity at follow-up. Cross-sectional findings showed that within the insula, patients with a relapse had higher GM volume than healthy controls at baseline, whereas there was no difference between patients with and without relapse. The same pattern was observed for the DLFPC. At follow-up, however, patients with relapse showed a lower gray matter volume compared to both patients without relapse and healthy controls (Figure 1).Discussion
Patients that experienced at least one relapse between scans showed a decline of insular and dorsolateral prefrontal gray matter volume over time. In patients without relapse, gray matter volume was stable. These results highlight the effects of patients’ course of illness on structural brain alterations in MDD. Although the direct link between structural and functional alterations has not been investigated yet, one might hypothesize that insular atrophy contributes to a selective mood-congruent activation towards emotional stimuli 7 and use of suppressive emotion regulation strategies 8. For the prefrontal cortex, meta-analyses frequently report gray matter alterations in patients with MDD 9 as well as in healthy controls with a familiar risk for MDD 10. In the present study, we unexpectedly observed larger DLPFC volume in patients with MDD than in healthy controls at baseline. As most of our MDD patients were medicated at baseline, these differences between patients and controls could be due to neuroprotective effects of antidepressants on gray matter volume. Relapse might boost gray matter decline, as already indicated by a study showing less progressed decrease in remitted subgroups after three years 11. Taken together, our present findings on insular and dorsolateral prefrontal atrophy in recurrent MDD shed light on the development of a bottom-up processing bias of emotional stimuli (e.g. in limbic brain areas) and a disruption of top-down executive functions (e.g. in the DLPFC).Conclusions
In sum, the present study revealed distinct effects of relapse in MDD using a longitudinal MRI design, which were neither associated with psychiatric medication nor with depression severity at follow-up. These results illustrate the negative effects of relapse on structural brain alterations and highlight the importance of improving prognosis and maintenance treatment in recurrent MDD.Acknowledgements
This work was funded by the German Research Foundation (DFG, grant FOR2107 DA1151/5-1 to UD; SFB-TRR58, Project C09 to UD) and the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (grant Dan3/012/17 to UD)References
1. Kessler RC, Berglund P, Demler O, et al. The Epidemiology of Major Depressive Disorder. JAMA 2003; 289(23):3095–3105. 2. Kanai T, Takeuchi H, Furukawa TA, et al. Time to recurrence after recovery from major depressive episodes and its predictors. Psychological Medicine 2003; 33(5):839–845. 3. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. PLoS Medicine 2013; 10(11):e1001547. 4. Stratmann M, Konrad C, Kugel H, et al. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PloS One 2014; 9(7):e102692. 5. www.neuro.uni-jena.de/cat, Version 933. 6. www.fil.ion.ucl.ac.uk/spm, Version 6685. 7. Stuhrmann A, Suslow T, Dannlowski U, et al. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biology of Mood & Anxiety Disorders 2011; 1(1):10. 8. Giuliani NR, Drabant EM, Bhatnagar R, Gross JJ. Emotion regulation and brain plasticity: Expressive suppression use predicts anterior insula volume. NeuroImage 2011; 58(1):10–15. 9. Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping 2009; 30(11):3719–3735. 10. Amico F, Meisenzahl EM, Koutsouleris N, et al. Structural MRI correlates for vulnerability and resilience to major depressive disorder. Journal of Psychiatry & Neuroscience 2011; 36(1):15–22. 11. Frodl T, Koutsouleris N, Bottlender R, et al. Depression-related variation in brain morphology over 3 years: effects of stress? Archives of General Psychiatry 2008; 65(10):1156–1165.Figures
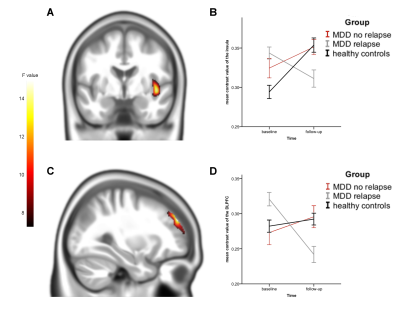
Figure 1. A:
Significant cluster of the group by time interaction in the right insular
cortex (y = -3), thresholded at p < .001, uncorrected. B: Mean contrast values of the right insular cortex for subjects
with relapse, without relapse and healthy controls at both time-points. C: Significant cluster of the group by
time interaction in the right DLPFC (x = 30), thresholded at p < .001,
uncorrected. D: Mean contrast values
of the right DLPFC for subjects with relapse, without relapse and healthy
controls at both time-points. Color bar, F values. Error bars, one standard
error of mean.