5308
A multi-methodological fMRI resting state voxel-wise analysis to assess brain abnormalities of children with ADHD1Huaxi Magnetic Resonance Research Centre (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, China, 2West China Hospital / West China School of Medicine Sichuan University, Chengdu, China
Synopsis
We used three different measurements including regional homogeneity (ReHo), voxel-mirrored homotopic connectivity (VMHC) and functional connectivity strength (FCS) to explore local and interhemispheric FC in drug naïve ADHD children. And we found lower ReHo and FCS in ADHD located in almost identical region of right middle frontal gyrus and correlated with each other. In addition, we also found lower VMHC in the bilateral occipital lobe, which was related with characteristics of WCST and CPRS-R. This finding may provide new insights into functional connectivity changes in ADHD and promote the exploration of functional network in the future.
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a common neuropsychiatric disorder in children and adolescents with a prevalence of 5.29% according to recent meta-analysis studies1. Resting-state functional magnetic resonance imaging (rs-fMRI) has played an increasingly important role in the elucidation of neurophysiological underpinnings in ADHD. Though quite a few studies had focused on the functional connectivity (FC) within different and distant regions using methods such as seed-based correlation (SBC), independent component analysis (ICA), relatively little known about the alterations in interhemispheric and more localized regional connectivities in ADHD. In the current study, we have therefore used three different measurements including regional homogeneity (ReHo) , voxel-mirrored homotopic connectivity (VMHC) and functional connectivity strength (FCS) to explore local and interhemispheric FC in a group of drug naïve ADHD children.METHODS
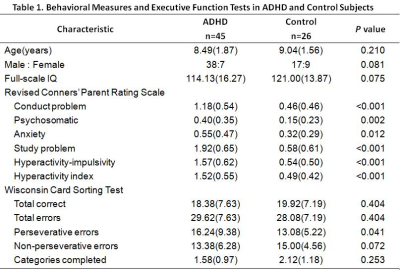
This study was approved by the local ethical committee, and written informed consent was obtained from guardians of all subjects. Forty-five medication-naive children diagnosed as ADHD according to DSM-V criterion and twenty-six sex and age matched healthy controls were included in the study. The revised Conners' Parent Rating Scale (CPRS-R) and Wisconsin Card Sorting Test (WCST) were used to assess behavioral measures and executive function respectively. All subjects underwent resting-state functional MR imaging by using a 3-T MR imaging system (Signal HDx, GE) with an eight-channel phased-array head coil. Resting state functional MR images were obtained using a gradient-echo echo-planar imaging sequence with the following parameters: 31 axial slices; slice thickness, 4mm; slice gap, 0.2mm, repetition time, 2000ms, echo time, 30ms, flip angle, 90°, matrix size, 64×64, field of view, 192×192mm2. Head translation movement for all participants was less than 2 mm, and rotation was less than 2°. The image data processing was conducted by REST software to calculate the parametric maps of ReHo, VMHC and whole brain FCS. Voxel-based analysis between patients and healthy controls was performed using two-sample t-test in SPM8. The p value of less than 0.005 at voxel-level and FWE-corrected p value of less than 0.05 at cluster-level were deemed to be significant. In the ADHD group, two-tailed Pearson correlation analyses were performed in SPSS 21.0 to assess the relationship between averaged eigenvalues of altered values (ReHo, VMHC and FCS) and characteristics of WCST and CPRS-R.RESULTS
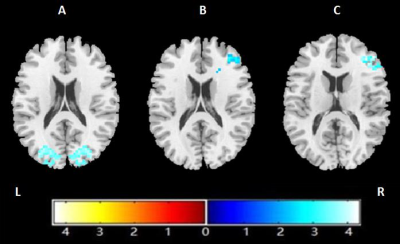
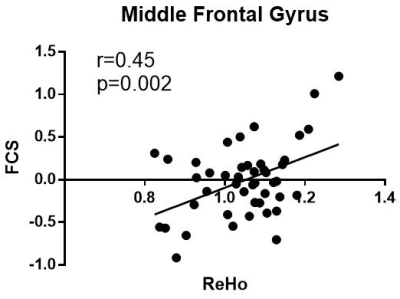
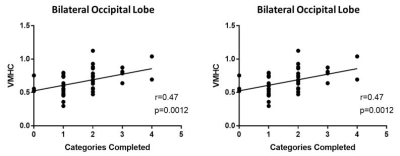
Relative to healthy control subjects, patients with ADHD showed both lower ReHo (cluster-level, p=0.011, FWE-corrected) and FCS (cluster level, p<0.001, FWE-corrected) in the right middle frontal gyrus (MFG); and lower VMHC in the bilateral occipital lobe (cluster-level, p=0.010, FWE-corrected) (Figure 1). The value of ReHo was positively correlated with FCS (Figure 2). The VMHC value located in the bilateral occipital lobe was positively correlated to patients’ anxiety scores of CPRS-R (r = 0.47, p = 0.0012), and inversely correlated to scores of completed categories of the WCST (r = -0.52, p < 0.001) in ADHD children group (Figure 3).No statistically significant correlation was found between the ReHo or FCS values and characteristics of WCST and CPRS-R.DISCUSSION&CONCLUSION
Interestingly, in current study we found the reduction of ReHo and FCS in ADHD located in almost identical region of right middle frontal gyrus and correlated with each other. This association between local connectivity and connectivity strength to the whole brain seems to suggest the important pathological role of this region in ADHD.
In addition, we also found lower VMHC in the bilateral occipital lobe, which was positively related with completed categories of the WCST and negatively related with anxiety scores of the CPRS-R. Diffusion tensor imaging studies had depicted decreased fractional anisotropy (FA) of the splenium of the corpus callosum (CC) in ADHD patients2, which is the major white matter bundle that connects bilateral occipital area3, influencing the speed of visual information transmission and dynamically distributing processing resources4. Thus, we speculated that the lower VMHC across the bilateral occipital lobe in ADHD found in current study might suggest deficits in visual circuit in AHDH which correlated with anxiety and distractibility.
Taken together, our work is expected to provide new insights into functional connectivity changes in ADHD and promote the exploration of functional network in the future.
Acknowledgements
No acknowledgement found.References
1.Polanczyk, G., et al. The Worldwide Prevalence of ADHD: A Systematic Review and Meta regression Analysis. American Journal of Psychiatry. 2007;164(6): 942.
2.Chen, L.Z., et al. A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2016. 68: 838-847.
3.Hofer, S. and J. Frahm. Topography of the human corpus callosum revisited - Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage.2006. 32(3): 989-994.
4.Putnam, M.C., et al. Cortical Projection Topography of the Human Splenium: Hemispheric Asymmetry and Individual Differences. Journal of Cognitive Neuroscience.2010. 22(8): 1662-1669.
Figures