5307
Multimodal metabolic imaging using single voxel MRS and [11C]ABP688 PET in Schizophrenic Patients1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Jülich, Jülich, Germany, 2Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Aachen, Germany, 3JARA – BRAIN – Translational Medicine, Jülich, Germany, 4TRIMAGE – consortium (http://www.trimage.eu/), Jülich, Germany, 5Institute of Neuroscience and Medicine 2, INM-2, Forschungszentrum Jülich, Jülich, Germany, 6Institute of Neuroscience and Medicine 5, INM-5, Forschungszentrum Jülich, Jülich, Germany, 7Department of Nuclear Medicine, RWTH Aachen University, Aachen, Germany, 8Institute of Neuroscience and Medicine 11, INM-11, Forschungszentrum Jülich, Jülich, Germany, 9Department of Neurology, RWTH Aachen University, Aachen, Germany, 10Monash Biomedical Imaging, School of Psychological Sciences, Monash University, Melbourne, Australia
Synopsis
By utilizing a multimodal imaging approach, the levels of glutamate and other metabolites in the anterior cingulate and the posterior cingulate cortex were assessed by MRS and compared to the metabotropic glutamate receptor 5 (mGluR5) binding potential using [11C]ABP688 PET in schizophrenic patients and healthy controls. Glutamate levels seem to be elevated in schizophrenic patients. PET analysis revealed no differences in binding potential (BPND) in both diagnostic groups. However, adding smoking status to analysis, there is a significant reduction in BPND in smokers compared to non-smokers, suggesting a connection between mGluR5 binding potential and nicotine dependence.
Introduction
Many cross-sectional studies implicate glutamatergic alterations in schizophrenia1,2, showing decreased glutamate levels in chronic and increased levels in medication free patients. A recent study showed that the metabotropic glutamate receptor 5 (mGluR5) modulates the NMDA receptor3, whose function is decreased in schizophrenia4,5. Thus, mGluR5 may also be directly involved in the abnormal glutamate signaling in schizophrenia. In this study, by utilizing a multimodal imaging approach6, the levels of glutamate (Glu) and other metabolites in the anterior cingulate (ACC) and the posterior cingulate cortex (PCC) were assessed by magnetic resonance spectroscopy (MRS) and compared to the mGluR5 binding potential using [11C]ABP688 ((E)-3-((6-methylpyridin-2-yl)ethynyl)-cyclohex-2-enone-O-11C-methyl-oxime) positron emission tomography (PET).Methods
Subjects
In one session per subject data were simultaneously acquired in a 3T MR-BrainPET system (Siemens, Germany)7 in four schizophrenic patients (mean age 37 ± 11.5) and four healthy volunteers (mean age 37.75 ± 11.8) matched for age, gender, smoker status, education and ethnical background.
PET data acquisition and analysis
[11C]ABP688 was injected (525 ± 55 MBq) as bolus + infusion (KBol=53min) while the volunteer was lying in the scanner. PET data acquisition (65 minutes) in list mode started simultaneously with the injection of the tracer. Data were iteratively reconstructed into 42 frames (30x10 s and 12x300 s time length) with 153 slices (voxel size 1.25 x 1.25 x 1.25 mm3, matrix size 256 x 256). Reconstruction incorporates corrections for attenuation, random and scattered coincidences, dead time and pile up. Smoothing (3mm Gaussian) and motion correction were applied. The period between 30 and 35 minutes was used for the calculation of non-displaceable binding potential (BPND). The BPND was calculated as (C-C’)/C, where C denotes the activity concentration in the volume of interest (PCC, ACC) and C’ denotes the activity concentration in an area of very low Glu receptor density (cerebellum).
MR data acquisition and analysis
MR data acquisition started simultaneously with PET acquisition. MPRAGE was used for structural MR imaging (TR=2250ms, TE=3.03ms, GRAPPA 2). Single voxel spectra were measured using standard point resolved spectroscopy (PRESS) sequence (TR=2500ms, TE1=14ms, TE2=105ms, number of averages=128, 25 x 25 x 25 mm3 voxel size, RF pulse centered at 2.4ppm, 16 step phase cycling). One extra complete phase cycle was measured without water suppression RF pulse. Spectra were analyzed using “LC Model” (6.3-0I), fitting from 0.2 to 4.0 ppm. The metabolite ratios (to creatine + phosphocreatine (Cr+PCr)) were extracted for ACC and PCC.
Results
MRS data
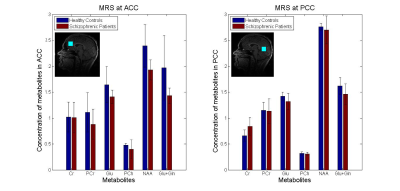
The metabolite levels in ACC and PCC are shown in Fig. 1. In both areas, there was no significant main effect of group in glutamate, although in ACC there is a trend that patients have lower glutamate levels. For N-acetylaspartate (NAA) the same trend can be seen.
PET data
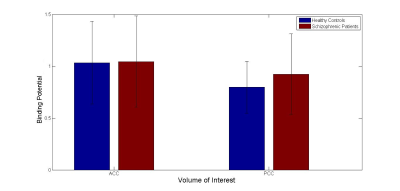
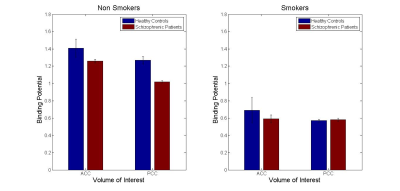
The data revealed no significant difference in mGluR5 binding potential between schizophrenia patients and healthy subjects in both ACC and PCC (Fig. 2). Adding the smoking status, the Wilcoxon-Mann-Whitney test showed a significant difference between smokers (n=4) and non-smokers (n=4) with no interaction with the diagnostic group in both regions (Z=1.94, p=0.05 (ACC); Z=2.17, p=0.03 (PCC)). The binding potential of both groups is shown in Fig. 3. However, a similar difference between smokers and non-smokers is not revealed in MRS data (data not shown).
Discussions
The trend to lower glutamate and NAA levels in schizophrenia patients is consistent with previous studies1 and may be associated with brain volume loss observed in schizophrenia patients8. Decreased glutamate levels as assessed via MRS could also be referred to NMDA receptor hypofunction observed in individuals with schizophrenia1,2. The reduction of binding potential in mGluR5 in smokers was also revealed in previous studies9,10. These findings suggest a relationship between down-regulation of mGluR5 and nicotine dependence. Most of the schizophrenic patients are smokers11. Since mGluR5 and NMDA receptors are closely connected, the down-regulation of mGluR5 could also contribute to NMDA hypofunction, which is thought to contribute to different schizophrenia symptoms5.Conclusions
The mGluR5 binding potential did not show differences between schizophrenia patients and healthy subjects. However, the glutamate concentration as assessed via MRS seems to be reduced in schizophrenia. Due to a close connection of NMDA receptor and mGluR5, it might demonstrate a target for novel treatments of schizophrenia. This exploratory feasibility study must be continued with a larger sample size to verify these metabolic changes in detail, especially the mismatch between MRS and PET.Acknowledgements
We gratefully thank Dr. Desmond Tse for optimizing MRS PRESS sequence. We thank Magdalene Vögeling, Cornelia Frey, Silke Frensch, and Suzanne Schaden for their technical assistance. This study was in part supported by the EU FP7 funded project TRIMAGE (Nr. 602621).References
1. Marsman A, van den Heuvel MP, Klomp DWJ, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull. 2011;39(1):120-129.
2. Poels EMP, Kegeles LS, Kantrowitz JT, et al. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res. 2014;152(2):325-332.
3. Matosin N, Fernandez-Enright F, Fung SJ, et al. Alterations of mGluR5 and its endogenous regulators Norbin, Tamalin and Preso1 in schizophrenia: towards a model of mGluR5 dysregulation. Acta Neuropathol. 2015;130(1):119-129.
4. Iwata Y, Nakajima S, Suzuki T, et al. Effects of glutamate positive modulators on cognitive deficits in schizophrenia: a systematic review and meta-analysis of double-blind randomized controlled trials. Mol Psychiatry. 2015;20(10):1151-1160.
5. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83(3):108-121.
6. Shah NJ, Arrubla J, Rajkumar R, et al. Multimodal Fingerprints of Resting State Networks as assessed by Simultaneous Trimodal MR-PET-EEG Imaging. Sci Rep. 2017;7(1):6452. doi:10.1038/s41598-017-05484-w.
7. Herzog H, Langen KJ, Weirich C, et al. High resolution BrainPET combined with simultaneous MRI. Nukl Med. 2011;50(2):74.
8. van Haren NEM, Pol HEH, Schnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63(1):106-113.
9. Akkus F, Ametamey S, Treyer V, et al. Marked Global Reduction in mGluR5 Binding in Smokers and Ex-smokers Determined by [11C] ABP688 Positron Emission Tomography. Proc Natl Acad Sci. 2012;110(2):737-742.
10. Akkus F, Treyer V, Ametamey SM, Johayem A, Buck A, Hasler G. Metabotropic glutamate receptor 5 neuroimaging in schizophrenia. Schizophr Res. 2017;183:95-101.
11. Wing VC, Wass CE, Soh DW, George TP. A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Ann N Y Acad Sci. 2012;1248(1):89-106.
Figures