5306
EEG Correlates of Real-time fMRI Neurofeedback of the Amygdala in Combat-related PTSD Evaluated Using eLORETA1Laureate Institute for Brain Research, Tulsa, OK, United States, 2Laureate Psychiatric Clinic and Hospital, Tulsa, OK, United States, 3Neuroscience Dept., George Mason University, Fairfax, VA, United States, 4Dept. of Psychological Science, University of Arkansas, Fayetteville, AR, United States, 5College of Engineering, Stephenson School of Biomedical Engineering, University of Oklahoma, Tulsa, OK, United States
Synopsis
We have performed a study of emotion regulation training in veterans with combat-related PTSD using real-time fMRI neurofeedback (rtfMRI-nf) with simultaneous EEG. Eighteen PTSD patients learned to upregulate their left amygdala activity using rtfMRI-nf during a positive emotion induction task based on retrieval of happy autobiographical memories. EEG source analysis with eLORETA revealed task-specific changes in the current source densities in the upper alpha and delta EEG bands that significantly correlated with PTSD severity. These results suggest that the rtfMRI-nf training in combination with EEG source analysis provides new insights into the neurobiology of PTSD.
Purpose:
Posttraumatic stress disorder (PTSD) causes abnormalities in brain regional and network activities1,2. Real-time fMRI neurofeedback (rtfMRI-nf) of the amygdala with simultaneous EEG3,4 allows modulation of the amygdala network activity and investigation of related EEG effects. Here we evaluate EEG correlates of rtfMRI-nf training of the left amygdala (LA) in veterans with combat-related PTSD4 using the exact low-resolution electromagnetic tomography (eLORETA)5,6. The participants learned to upregulate the LA activity with rtfMRI-nf while performing a positive emotion induction task based on retrieval of happy autobiographical memories4. Our EEG source analysis reveals task-specific changes in the current source densities in the upper alpha (α2) and delta (δ) EEG bands that significantly correlate with PTSD severity.Methods:
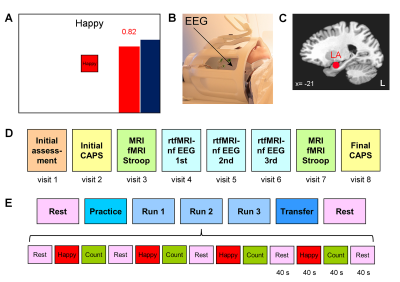
Twenty male patients with a primary diagnosis of PTSD related to combat trauma participated in the study and completed the first training session (Fig. 1D, visit 4) involving rtfMRI-nf with simultaneous EEG. Clinical assessment included the Clinician-Administered PTSD Scale for DSM-IV (CAPS), the Hamilton Depression Rating Scale (HDRS), and other instruments. Data from 18 patients were included in the group analyses.
The experiments were performed on a GE MR750 3T MRI scanner with an 8-channel receive-only head coil. A gradient echo EPI sequence with FOV/slice=240/2.9 mm, TR/TE=2000/30 ms, SENSE R=2, image matrix 96×96, flip=90°, 34 axial slices, was employed for fMRI. Simultaneous EEG recordings were performed using a 32-channel MR-compatible EEG system (Brain Products GmbH) in 0.016−250 Hz band with 0.1 µV resolution and 5 kS/s sampling. The rtfMRI-nf was implemented using a custom real-time system with a neurofeedback GUI (Fig. 1A)3,4. The nf signal was based on fMRI activation in the LA target ROI (Fig. 1C)3,4. The rtfMRI-nf session protocol (Fig. 1E) included seven runs, and each run (except Rest) consisted of 40-s blocks of Rest, Happy Memories, and Count conditions. For each Happy Memories with rtfMRI-nf condition, the participant was asked to feel happy by evoking happy autobiographical memories, while trying to raise the level of the red rtfMRI-nf bar to that of the blue target bar (Fig. 1A). During the Transfer run, the participant followed the same procedure, but no bars were shown on the screen3,4.
EEG data processing was performed using BrainVision Analyzer 2 software (Brain Products, GmbH). EEG-fMRI artifacts were removed using the average artifact subtraction and ICA. EEG source analysis was conducted using LORETA-KEY software (KEY Institute for Brain-Mind Research). The analysis was applied to EEG data from 30 channels with common average reference. It involved computation of EEG cross-spectra separately for Happy Memories and Rest conditions in each run. The cross-spectra were computed for the standard EEG frequency bands in the LORETA-KEY software, including the upper alpha band α2 [10.5...12] Hz and the delta band δ [1.5...6] Hz. The current source density was obtained using the eLORETA transform. Group analysis involved voxel-wise comparison of the current density values between the Happy Memories and Rest conditions and included the subjects’ PTSD severity ratings (CAPS) as a covariate. Current density values for Brodmann area (BA) centroids (i.e. single voxels at Brodmann areas’ centers of mass) were exported and used in partial correlation analyses.
Results:
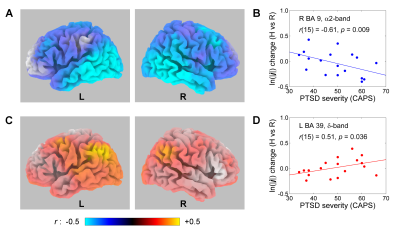
During the Transfer run, the PTSD veterans demonstrated significant fMRI activation of the LA. The Happy Memories vs Rest current density changes in the upper alpha (α2) band showed significant negative correlations with PTSD severity across the temporal and parietal lobes (Fig. 2A) and for the right DLPFC (BA 9/8/45/46), as illustrated in Fig. 2B. The corresponding current density changes in the delta (δ) band exhibited significant positive correlations with PTSD severity for the parietal regions (BA 39/40, Fig. 2C), as illustrated in Fig. 2D.Discussion:
Patients with more severe PTSD (higher CAPS) showed stronger activation (reduction in α2 activity) of the temporal and parietal brain regions (Fig. 2A), involved in emotion processing and episodic memory retrieval, during the Transfer run following the rtfMRI-nf training. This means that hyperactivity of these regions in PTSD is observed not only during recollection of traumatic events1,2, but also during recall of happy autobiographical memories. This conclusion is further supported by the higher δ activity in the parietal regions (Fig. 2C), involved in autobiographical memory retrieval, in patients with higher PTSD severity. The increased activation (reduced α2 activity) of the right DLPFC (Fig. 2A,B) in PTSD is consistent with previous observations7 and indicates reduced frontal EEG asymmetry during an emotional challenge in PTSD8. Overall, the rtfMRI-nf training in combination with EEG source analysis provides new insights into the neurobiology of PTSD.Acknowledgements
This research was supported by W81XWH-12-1-0697 grant from the US Department of Defense.References
1. Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476-1488.
2. Lanius RA, Bluhm R, Lanius U, et al. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiat Res 2006; 40:709-729.
3. Zotev V, Yuan H, Misaki M, et al. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. NeuroImage Clin 2016; 11:224-238.
4. Zotev V, Phillips R, Misaki M, et al. Real-time fMRI neurofeedback of the amygdala enhances amygdala-orbitofrontal connectivity and lateralized EEG coherence in veterans with combat-related PTSD. ISMRM Proceedings 2017; 0512.
5. Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 1994; 18:49-65.
6. Pascual-Marqui RD, Lehmann D, Koukkou M, et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Phil Trans R Soc A 2011; 369:3768-3784.
7. Rabe S, Zoellner T, Beauducel A, et al. Changes in brain electrical activity after cognitive behavioral therapy for posttraumatic stress disorder in patients injured in motor vehicle accidents. Psychosom Med 2008; 70:13-19.
8. Meyer T, Smeets T, Giesbrecht T, et al. The role of frontal EEG asymmetry in post-traumatic stress disorder. Biol Psychol 2015; 108:62-77.
Figures