5304
Whole brain effect of real-time fMRI amygdala neurofeedback emotional training and its association with PTSD symptom reduction1Laureate Institute for Brain Research, Tulsa, OK, United States, 2Laureate Psychiatric Clinic and Hospital, Tulsa, OK, United States, 3George Mason University, Fairfax, VA, United States, 4University of Arkansas, Fayetteville, AR, United States, 5University of Oklahoma, Norman, OK, United States
Synopsis
The effect of real-time fMRI neurofeedback (rtfMRI-nf) training with the left amygdala activity on whole brain regions and their association with symptom reduction was investigated in veterans with combat-related PTSD. The main effect of training was seen in the salience network regions including anterior insula and the anterior cingulate cortex (ACC). The decrease in ACC response was significantly correlated with a decrease in PTSD symptoms. These results indicated that the effect of rtfMRI-nf training was not limited to the left amygdala but other emotion-related regions were co-modulated during the training. The treatment response could be meditated by those regions.
Introduction
Real-time fMRI neurofeedback (rtfMRI-nf) enables self-regulating brain activity. Emerging evidence suggests its clinical utility in treating psychiatric disorders1-3. However, not all participants are successful in brain self-regulation and show a response to the treatment. This study aimed to examine factors that were associated with rtfMRI-nf training success in increasing left amygdala (LA) activity during positive autobiographical memory recall and decrease in symptoms as a result of the training for combat-related posttraumatic stress disorder (PTSD) patients.Methods
Thirty-six male combat veterans with PTSD (age 21-48, mean=32, SD=7) participated in 3-days rtfMRI-nf training sessions. The participants were randomly divided into the experimental group (PTSD-exp, N=25) who received a feedback signal from the left amygdala (LA) and the control group (PTSD-ctrl, N=11) who received a feedback signal from a non-emotion-related region (the left horizontal segment of the intraparietal sulcus). Participants were instructed to retrieve positive memories while attempting to increase the feedback signal presented on a screen via a bar. Each neurofeedback runs consisted of alternating 40s blocks of rest, feedback (happy memory retrieval), and count (backward from 300 by a given one-digit integer) conditions. Each training session consisted of four fMRI runs, each lasting 8min 46s; a practice run, three training runs, and a final transfer run in which no neurofeedback information was provided. Training sessions were separated about a week and a pre and post assessment of PTSD symptoms (the Clinician-Administered PTSD Scale [CAPS] for DSM-IV4) was performed about two weeks before and after the training.
AFNI (http://afni.nimh.nih.gov/afni/) was used for image processing. GLM analysis was performed with happy and count block regressors as well as noise regressors of 3 principal components of ventricle signal, local white matter average signal (ANATICOR), motion parameters, and low-frequency fluctuation. Beta-value of the happy block regressor was extracted as brain response for individual participants. Group analysis was performed with linear mixed-effect model (LME) analysis to examining the effects of training runs, sessions, and groups on happy block brain response in LA region of interest (ROI) and whole brain analyses.
Results
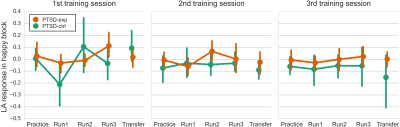
CAPS total score significantly decreased after the training for PTSD-exp (p<0.001) but not for PTSD-ctrl (p=0.085). In the LA ROI analysis, while we observed increased LA activity at several runs, no significant effect of runs (p=0.108) and sessions (p=0.790) were seen in LME analysis (Fig. 1). We also found that LA response trend across runs was correlated with baseline (Practice run) LA response amplitude. Participants with low LA response at baseline showed a larger increase of LA response across runs (the main effect of baseline response on response trend was significant with LME analysis, p=0.004). LA response change was not associated with a change in CAPS total score (p=0.459).
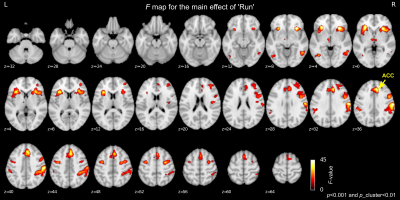
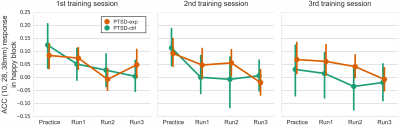
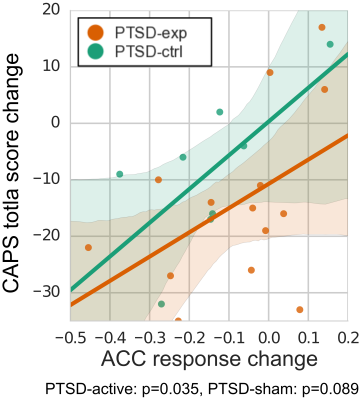
Whole brain LME analysis revealed significant main effects of runs and sessions in the lateral frontal regions, postcentral regions, and the salience network regions including anterior insula and anterior cingulate cortex (ACC) (voxel-wise p<0.001 and cluster-size5 p<0.01, Fig. 2). The salience network regions showed a decreasing trend across runs and sessions (Fig. 3). The decrease in the ACC response was significantly correlated with a decrease in CAPS total score (p=0.035 for PTSD-exp and p=0.089 for PTSD-ctrl, Fig.4).
Discussion
While rtfMRI-nf training could increase LA response, there was no consistent increasing trend across multiple runs and sessions. We found, however, that LA response trend associated with baseline LA response. In addition, PTSD symptom change was not associated with a change in LA response. This suggests that while rtfMRI-nf helped maintain increased LA activity during positive memory recall especially for participants who could not increase LA response at baseline, LA response did not mediate symptom reduction. Significant training effect across runs and sessions was seen in the salience network regions whose response decreased across training. Furthermore, there was a significant association between PTSD symptom reduction and the decrease in the ACC response. These indicate that rtfMRI-nf training effect was not limited to the feedback target region (i.e. LA), but the training could affect other brain regions. The result suggests that the observed PTSD symptom reduction could be mediated by the co-activated brain regions (i.e. ACC).Acknowledgements
This research was supported by W81XWH-12-1-0697 award from the U.S. Department of Defense, the Laureate Institute for Brain Research (LIBR), and the William K. Warren Foundation.References
1. Linden, D.E.J., I. Habes, S.J. Johnston, et al., Real-Time Self-Regulation of Emotion Networks in Patients with Depression. PLoS ONE, 2012;7(6):e38115.
2. Zilverstand, A., B. Sorger, P. Sarkheil, et al., fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front Behav Neurosci, 2015;9:148.
3. Young, K.D., G.J. Siegle, V. Zotev, et al., Randomized Clinical Trial of Real-Time fMRI Amygdala Neurofeedback for Major Depressive Disorder: Effects on Symptoms and Autobiographical Memory Recall. Am J Psychiatry, 2017;174(8):748-755.
4. Blake, D., F. Weathers, L. Nagy, et al., Clinician-Administered PTSD Scale for DSM-IV (CAPS-DX). National Center for Posttraumatic Stress Disorder, Behavioral Science Division, Boston VA Medical Center, Boston, MA, 1995.
5. Cox, R.W., G. Chen, D.R. Glen, et al., FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect, 2017;7(3):152-171.
Figures