5297
Ex-vivo diffusion MRI reveals microstructural alterations in stress-sensitive brain regions: A chronic mild stress recovery study1Clinical Medicine, Aarhus University, Aarhus C, Denmark, 2Oregon Health and Science University, Portland, OR, United States, 3Dept. of Physics and Astronomy, Aarhus University, Aarhus, Denmark
Synopsis
Depression is a leading cause of disability worldwide and causes significant microstructural alterations in stress-sensitive brain regions. However, the potential recovery of these microstructural alterations has not previously been investigated, which we, therefore, set out to do using diffusion MRI (d-MRI) in the chronic mild stress (CMS) rat model of depression. This study reveals significant microstructural alterations after 8 weeks of recovery, in the opposite direction to change induced by stress in the acute phase of the experiment. Such findings may be useful in the prognosis of depression or for monitoring treatment response.
Introduction
Depression is one of the largest single causes of non-fatal burden1. Despite its worldwide prevalence, no single biomarker is established to diagnose depression, partly due to the immense heterogeneity of symptoms2. However, stress sensitive regions of the brain have shown consistent alterations, such as dendritic atrophy in the hippocampus (HP), prefrontal cortex (PFC), caudate putamen (CP) and hypertrophy in the amygdala (AM)3.Methods to monitor recovery of these microstructural alterations in stress-sensitive brain regions are lacking but could be very useful in disease prognosis and in monitoring treatment. The present study employed the unpredictable chronic mild stress (CMS) model of depression in rats to examine diffusion MRI (d-MRI) informed microstructural alterations in stress-sensitive brain regions after 8 weeks of CMS recovery. The study shows significant microstructural alterations in the dorsal and ventral hippocampus (dHP and vHP), CP, and AM in the anhedonic group in comparison to control.Material and Methods
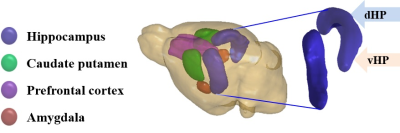
All experimental procedures were performed according to institutional and ethical committee guidelines. Long Evans rats were exposed to an eight weeks long unpredictable CMS paradigm and were identified as anhedonic and resilient on the basis of sucrose consumption testing2. Only the anhedonic group (N=8) was considered for recovery for 8 weeks after the CMS paradigm and compared to controls (N=8). After eight weeks of recovery, all rats were euthanized and brains were extracted after perfusion fixation. Ex-vivo MRI of the left-brain hemisphere was performed on a 9.4T preclinical MRI system (Bruker, Germany). An EPI-DTI sequence was employed to acquire the d-MRI data with parameters TR/TE = 2500/26.7 ms, δ/Δ= 6/14 ms, 8 b-values with 25 directions. A subset of d-MRI data (up to a b-value 2.5ms/μm2) was utilized for diffusion tensor parameters: fractional anisotropy (FA), mean diffusivity(MD), axial diffusivity(AD), and radial diffusivity (RD), axisymmetric kurtosis parameters: mean kurtosis (MK), axial kurtosis (AK), and radial kurtosis (RK)4, and fast kurtosis parameters: mean kurtosis tensor (MKT), axial kurtosis tensor (WL), and radial kurtosis tensor (WT)4, 5. Regions of interest (ROIs) (Figure 1) were drawn on the b0 images of the d-MRI data of each brain and imported to the computed maps of diffusion tensor and kurtosis parameters. Student's t-tests were applied to evaluate the level of significance between the groups.Results
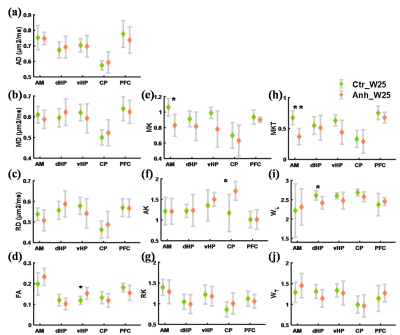
Among the diffusion tensor parameters, FA was significantly higher in the vHP (p<0.05) of the anhedonic group after 8 weeks of recovery in comparison to control. The axisymmetric kurtosis parameter MK was significantly lower in the AM (p<0.05) and significantly higher AK was observed in the CP (p<0.05) compared to controls. The fast kurtosis parameter MKT was significantly lower in the AM (p<0.01) as was WL in dHP (p<0.05) in comparison to controls (Figure 2).Discussion
The d-MRI based kurtosis parameters MK and MKT showed a significant decrease in the AM, possibly indicating over normalization of hyperactive AM as observed in the acute phase of post CMS paradigm in the previous studies3, 6. The increase in FA in vHP and decrease in WL in dHP also support hippocampal susceptibility towards stress and shows differential microstructural alterations in the dHP and vHP7.Immuno-histochemistry data may provide specific microstructural changes, such as axons and dendritic alterations in the hippocampus as well as in the other regions, to reveal the neurobiological underpinnings of our d-MRI findings. A recent study found a significantly higher AD in the CP, but no change in the kurtosis parameters after CMS paradigm while the present study shows a significant increase in AK in the CP region but not in the diffusion tensor parameters. This could indicate an incomplete recovery of the region.Conclusion
This study found significant d-MRI based microstructural alterations in the stress circuitry after eight weeks of recovery from CMS. Further substantiation of these findings could be provided by immuno-histochemical analysis data acquired at additional time points. Such studies indicate the value of d-MRI to provide prognostic information in the recovery from depression.Acknowledgements
Lundbeck Foundation R83-A7548 and R253-2017-215.Danish Ministry of Science, Technology and Innovation’s University Investment Grant (MIND Lab, Grant no. 0601-01354B), and NIH 1R01EB012874-01. Danish Research Council's Infrastructure program, the Velux Foundations, and the Department of Clinical Medicine, AU.References
1. Ferrari, A.J., et al., Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. Plos one, 2013: p. e1001547.
2. Wiborg, O., Chronic mild stress for modeling anhedonia. Cell and tissue research, 2013. 354(1): p. 155-169.
3. Khan, A.R., et al., Biophysical modeling of high field diffusion MRI demonstrates micro-structural aberration in chronic mild stress rat brain. Neuroimage, 2016. 142: p. 421-430.
4. Hansen, B., N. Shemesh, and S.N. Jespersen, Fast imaging of mean, axial and radial diffusion kurtosis. Neuroimage, 2016. 142: p. 381-393.
5. Hansen, B., et al., Experimentally and computationally fast method for estimation of a mean kurtosis. Magn Reson Med, 2013. 69(6): p. 1754-60.
6.Vyas, A., S. Bernal, and S. Chattarji, Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain research, 2003. 965(1): p. 290-294.
7. Bannerman, D., et al., Regional dissociations within the hippocampus—memory and anxiety. Neuroscience & Biobehavioral Reviews, 2004. 28(3): p. 273-283.
8. Paxinos, G. and C. Watson, The rat brain in stereotaxic coordinates. Qingchuan Zhuge translate)(People’s Medical Publishing House, Beijing, China, 2007), 1998. 32.
Figures